Abstract
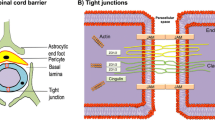
After spinal cord injury (SCI), tight junction (TJ) protein degradation increases permeability and disrupts the blood–spinal cord barrier (BSCB). The BSCB is primarily formed of endothelial cell, which forms a specialized tight seal due to the presence of TJs. BSCB disruption after SCI allows neutrophil infiltration. Matrix metalloproteinase (MMP)-8 is believed to be mainly expressed by neutrophils and is quickly released upon neutrophil activation. Here, we determined whether MMP-8 is involved in the TJ protein degradation in endothelial cells and also determined its role in the neuroinflammation after SCI. MMP-8 recombinant protein treatment increases the TNF-α expression and decreased the TJ (occludin and zonula occludens-1) protein expression in the endothelial cells. Likewise, specific MMP-8 inhibitor (MMP-8I) significantly prevented the TNF-α-induced decrease in the expression of TJ protein in endothelial cells. Furthermore, MMP-8 expression was significantly increased 1 and 3 days after moderate compression (35 g for 5 min at T10 level) SCI, whereas TJ protein levels decreased as determined qRT-PCR, western blotting, and immunohistochemistry. MMP-8 was inhibited directly using a MMP-8I (5 mg/kg) and indirectly by reducing neutrophil infiltration with sivelestat sodium (50 mg/kg) or using the antioxidant N-acetyl-l-cysteine (100 mg/kg). The MMP-8I significantly decreased TNF-α expression, IL-6, and iNOS expression and increased TJ protein expression after SCI. In addition, MMP-8I significantly lessens the amount of Evans blue dye extravasation observed after injury. Thus, our result suggests that MMP-8 plays an imperative role in inflammation and degradation of TJ proteins. Increased MMP-8 expression was associated with the early inflammatory phase of SCI. Inhibiting MMP-8 significantly attenuated SCI-induced inflammation, BSCB breakdown, and cell injury.





Similar content being viewed by others
References
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? The Journal of Neuroscience: the official journal of the Society for Neuroscience 27(44):11782–11792. doi:10.1523/JNEUROSCI.3444-07.2007
Kumar H, Ropper AE, Lee SH, Han I (2016) Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol. doi:10.1007/s12035-016-9910-6
Bazzoni G, Dejana E (2004) Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84(3):869–901. doi:10.1152/physrev.00035.2003
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. doi:10.1124/pr.57.2.4
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. doi:10.1016/j.neuron.2008.01.003
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. doi:10.1038/nrn1824
Mautes AE, Weinzierl MR, Donovan F, Noble LJ (2000) Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 80(7):673–687
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L (1998) Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151(1):77–88. doi:10.1006/exnr.1998.6785
Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, Lecuyer MA, Alvarez JI et al (2014) Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol 193(5):2438–2454. doi:10.4049/jimmunol.1400401
Aslam M, Ahmad N, Srivastava R, Hemmer B (2012) TNF-alpha induced NFkappaB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine 57(2):269–275. doi:10.1016/j.cyto.2011.10.016
Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z et al (2014) Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A 111(11):E1035–E1042. doi:10.1073/pnas.1401595111
Sharma HS (2011) Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm 118(1):155–176. doi:10.1007/s00702-010-0514-4
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516. doi:10.1146/annurev.cellbio.17.1.463
Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ et al (1999) The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98(2):137–146
Werb Z (1997) ECM and cell surface proteolysis: regulating cellular ecology. Cell 91(4):439–442
Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z (2002) Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. The Journal of Neuroscience: the official journal of the Society for Neuroscience 22(17):7526–7535
Rosenberg GA, Estrada EY, Dencoff JE (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke; a journal of cerebral circulation 29(10):2189–2195
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108(3):811–820. doi:10.1111/j.1471-4159.2008.05821.x
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23(1):87–96. doi:10.1016/j.nbd.2006.02.006
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. The Journal of Neuroscience: the official journal of the Society for Neuroscience 21(19):7724–7732
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. Journal of Cerebral Blood Flow and Metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 27(4):697–709. doi:10.1038/sj.jcbfm.9600375
Buhler LA, Samara R, Guzman E, Wilson CL, Krizanac-Bengez L, Janigro D, Ethell DW (2009) Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci 10:17. doi:10.1186/1471-2202-10-17
Caron A, Desrosiers RR, Beliveau R (2005) Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9. Exp Cell Res 310(1):105–116. doi:10.1016/j.yexcr.2005.07.004
Van Lint P, Libert C (2006) Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev 17(4):217–223. doi:10.1016/j.cytogfr.2006.04.001
Lee EJ, Han JE, Woo MS, Shin JA, Park EM, Kang JL, Moon PG, Baek MC et al (2014) Matrix metalloproteinase-8 plays a pivotal role in neuroinflammation by modulating TNF-alpha activation. J Immunol 193(5):2384–2393. doi:10.4049/jimmunol.1303240
Dejonckheere E, Vandenbroucke RE, Libert C (2011) Matrix metalloproteinase8 has a central role in inflammatory disorders and cancer progression. Cytokine Growth Factor Rev 22(2):73–81. doi:10.1016/j.cytogfr.2011.02.002
Vandenbroucke RE, Dejonckheere E, Van Lint P, Demeestere D, Van Wonterghem E, Vanlaere I, Puimege L, Van Hauwermeiren F et al (2012) Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. The Journal of Neuroscience: the official journal of the Society for Neuroscience 32(29):9805–9816. doi:10.1523/JNEUROSCI.0967-12.2012
Ropper AE, Zeng X, Anderson JE, Yu D, Han I, Haragopal H, Teng YD (2015) An efficient device to experimentally model compression injury of mammalian spinal cord. Exp Neurol 271:515–523. doi:10.1016/j.expneurol.2015.07.012
Lee JY, Choi HY, Na WH, Ju BG, Yune TY (2014) Ghrelin inhibits BSCB disruption/hemorrhage by attenuating MMP-9 and SUR1/TrpM4 expression and activation after spinal cord injury. Biochim Biophys Acta 1842(12 Pt A):2403–2412. doi:10.1016/j.bbadis.2014.09.006
Löffek S, Schilling O, Franzke C-W (2011) Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38(1):191–208
Lee SM, Rosen S, Weinstein P, van Rooijen N, Noble-Haeusslein LJ (2011) Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J Neurotrauma 28(9):1893–1907. doi:10.1089/neu.2011.1860
Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S (2014) Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation 11(1):150
Faurschou M, Borregaard N (2003) Neutrophil granules and secretory vesicles in inflammation. Microbes and Infection / Institut Pasteur 5(14):1317–1327
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6(3):173–182. doi:10.1038/nri1785
Nygardas PT, Hinkkanen AE (2002) Up-regulation of MMP-8 and MMP-9 activity in the BALB/c mouse spinal cord correlates with the severity of experimental autoimmune encephalomyelitis. Clin Exp Immunol 128(2):245–254
Light M, Minor KH, DeWitt P, Jasper KH, Davies SJ (2012) Multiplex array proteomics detects increased MMP-8 in CSF after spinal cord injury. J Neuroinflammation 9:122. doi:10.1186/1742-2094-9-122
Moghaddam A, Heller R, Daniel V, Swing T, Akbar M, Gerner HJ, Biglari B (2016) Exploratory study to suggest the possibility of MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury. Spinal Cord. doi:10.1038/sc.2016.104
Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD et al (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129(12):3249–3269
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209(2):378–388
Acarin L, Gonzalez B, Castellano B (2000) Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur J Neurosci 12(10):3505–3520
Bartholdi D, Schwab ME (1997) Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 9(7):1422–1438
Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E (2000) Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 17(3):203–218
Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y (1996) Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol 55(3):280–289
Probert L, Selmaj K (1997) TNF and related molecules: trends in neuroscience and clinical applications. J Neuroimmunol 72(2):113–117
Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G (1997) TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol 72(2):137–141
Davies AL, Hayes KC, Dekaban GA (2007) Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 88(11):1384–1393. doi:10.1016/j.apmr.2007.08.004
Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J et al (1999) Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma 16(10):851–863. doi:10.1089/neu.1999.16.851
Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K (2000) Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res 85(1–2):114–122
Pearse DD, Chatzipanteli K, Marcillo AE, Bunge MB, Dietrich WD (2003) Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J Neuropathol Exp Neurol 62(11):1096–1107
Maggio DM, Chatzipanteli K, Masters N, Patel SP, Dietrich WD, Pearse DD (2012) Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J Neurotrauma 29(12):2244–2249. doi:10.1089/neu.2012.2371
Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, Iwamoto Y, Yoshizaki K et al (2004) Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res 76(2):265–276. doi:10.1002/jnr.20044
Thirkettle S, Decock J, Arnold H, Pennington CJ, Jaworski DM, Edwards DR (2013) Matrix metalloproteinase 8 (collagenase 2) induces the expression of interleukins 6 and 8 in breast cancer cells. J Biol Chem 288(23):16282–16294
Mautes AE, Bergeron M, Sharp FR, Panter SS, Weinzierl M, Guenther K, Noble LJ (2000) Sustained induction of heme oxygenase-1 in the traumatized spinal cord. Exp Neurol 166(2):254–265. doi:10.1006/exnr.2000.7520
Liu Y, Tachibana T, Dai Y, Kondo E, Fukuoka T, Yamanaka H, Noguchi K (2002) Heme oxygenase-1 expression after spinal cord injury: the induction in activated neutrophils. J Neurotrauma 19(4):479–490. doi:10.1089/08977150252932424
Mautes AE, Kim DH, Sharp FR, Panter S, Sato M, Maida N, Bergeron M, Guenther K et al (1998) Induction of heme oxygenase-1 (HO-1) in the contused spinal cord of the rat. Brain Res 795(1–2):17–24
Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ (2004) Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma 21(8):1017–1030. doi:10.1089/0897715041651042
Lin W-P, Xiong G-P, Lin Q, Chen X-W, Zhang L-Q, Shi J-X, Ke Q-F, Lin J-H (2016) Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J Neuroinflammation 13(1):1
Eissa N, Bhattacharya A (2014) Autophagy is required for neutrophil-mediated inflammation. In: D17. Stop, drop, and die: proteostasis and autophagy in the lung. Am Thoracic Soc, pp A5396-A5396
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 36(22):E1427–E1434. doi:10.1097/BRS.0b013e3182028c3a
Chen H-C, Fong T-H, Lee A-W, Chiu W-T (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine 37(6):470–475
Aikawa N, Kawasaki Y (2014) Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag 10:621–629. doi:10.2147/TCRM.S65066
Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E et al (2001) A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem 78(5):1064–1072
Sadowska AM, Manuel-y-Keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, De Backer WA (2006) Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: In vivo and in vitro study. Pharmacol Res 53(3):216–225. doi:10.1016/j.phrs.2005.11.003
Schubert-Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M (2010) Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog 6(4):e1000874. doi:10.1371/journal.ppat.1000874
Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA (2000) Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis 31(1):80–84. doi:10.1086/313922
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5(4):261–270. doi:10.1038/nrm1357
Acknowledgments
This work was supported by a grant of the National Research Foundation of Korea (NRF) (NRF-2014R1A1A2059118, NRF-2015H1D3A1066543), the Ministry of Science, ICT & Future Planning (NRF-2016R1A2A1A05004987), and the Korea Healthcare Technology Research & Development Project, Ministry for Health & Welfare Affairs, Republic of Korea (HI14C3270, HR16C0002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Fig. 1
Matrix metalloproteinase-8 (MMP-8) expression increases after spinal cord injury (SCI) at the epicenter of the damage. The spinal cord was collected 1, 3, 7, 14, 21 and 28 days (1D, 3D, 7D, 14D, 21D and 28D, respectively) after SCI to determine the expression of MMP-8 (A). Luxol fast blue (LFB) staining was performed at the epicenter of the SCI damage and in sham-injured rats 3H and 1D, 3D, 5D, and 7D, respectively after SCI in longitudinal section (B). (JPEG 86 kb).
Rights and permissions
About this article
Cite this article
Kumar, H., Jo, MJ., Choi, H. et al. Matrix Metalloproteinase-8 Inhibition Prevents Disruption of Blood–Spinal Cord Barrier and Attenuates Inflammation in Rat Model of Spinal Cord Injury. Mol Neurobiol 55, 2577–2590 (2018). https://doi.org/10.1007/s12035-017-0509-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0509-3