Abstract
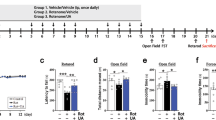
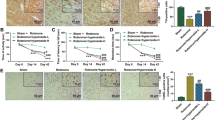
The recent emergence of ursodeoxycholic acid (UDCA) as a contender in modifying neurotoxicity in human dopaminergic cells as well as its recognized anti-apoptotic and anti-inflammatory potentials in various hepatic pathologies raised impetus in investigating its anti-parkinsonian effect in rat rotenone model. UDCA prominently improved motor performance in the open field test and halted the decline in the striatal dopamine content. Meanwhile, it improved mitochondrial function as verified by elevation of ATP associated with preservation of mitochondrial integrity as portrayed in the electron microscope examination. In addition, through its anti-inflammatory potential, UDCA reduced the rotenone-induced nuclear factor-κB expression and tumor necrosis factor alpha level. Furthermore, UDCA amended alterations in Bax and Bcl-2 and reduced the activities of caspase-8, caspase-9, and caspase-3, indicating that it suppressed rotenone-induced apoptosis via modulating both intrinsic and extrinsic pathways. In conclusion, UDCA can be introduced as a novel approach for the management of Parkinson’s disease via anti-apoptotic and anti-inflammatory mechanisms. These effects are probably linked to dopamine synthesis and mitochondrial regulation.




Similar content being viewed by others
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Phani S, Loike JD, Przedborski S (2012) Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S207–S209
Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A (2007) Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? Int Rev Neurobiol 82:235–246
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65(2):135–172
Tatton WG, Chalmers-Redman R, Brown D, Tatton N (2003) Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol 53(3):S61–S70, discussion S70-62
Danielson SR, Andersen JK (2008) Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radic Biol Med 44(10):1787–1794
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62(5):803–819
del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ (2000) Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 10(1):95–112
Liu WG, Chen Y, Li B, Lu GQ, Chen SD (2004) Neuroprotection by pergolide against levodopa-induced cytotoxicity of neural stem cells. Neurochem Res 29(12):2207–2214
Keeney PM, Xie J, Capaldi RA, Bennett JP Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26(19):5256–5264
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54(3):823–827
Braak H, Braak E (2000) Pathoanatomy of Parkinson’s disease. J Neurol 247(Suppl 2):II3–II10
Roma MG, Toledo FD, Boaglio AC, Basiglio CL, Crocenzi FA, Sanchez Pozzi EJ (2011) Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications. Clin Sci (Lond) 121(12):523–544
Parry GJ, Rodrigues CM, Aranha MM, Hilbert SJ, Davey C, Kelkar P, Low WC, Steer CJ (2010) Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol 33(1):17–21
Yanovsky Y, Schubring SR, Yao Q, Zhao Y, Li S, May A, Haas HL, Lin JS, Sergeeva OA (2012) Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS One 7(8):e42512
Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM (2009) Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res 50(9):1721–1734
Paumgartner G, Beuers U (2002) Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 36(3):525–531
Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ (1998) Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med 4(3):165–178
Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ (1999) Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ 6(9):842–854
Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, Cuccurullo F (2002) Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol 64(11):1661–1667
Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, Morimoto C, Makino I, Tanaka H (2001) Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem 276(50):47371–47378
Liu J, Zhou CX, Zhang ZJ, Wang LY, Jing ZW, Wang Z (2012) Synergistic mechanism of gene expression and pathways between jasminoidin and ursodeoxycholic acid in treating focal cerebral ischemia-reperfusion injury. CNS Neurosci Ther 18(8):674–682
Rodrigues CM, Sola S, Silva R, Brites D (2000) Bilirubin and amyloid-beta peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol Med 6(11):936–946
Vang S, Longley K, Steer CJ, Low WC (2014) The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Global Adv Health Med 3(3):58–69
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278(10):8516–8525
Swarnkar S, Goswami P, Kamat PK, Gupta S, Patro IK, Singh S, Nath C (2012) Rotenone-induced apoptosis and role of calcium: a study on Neuro-2a cells. Arch Toxicol 86(9):1387–1397
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324
Abd-El Gawad HM, Abdallah DM, El-Abhar HS (2004) Rotenone-induced Parkinson’s like disease: modulating role of coenzyme Q10. J Biol Sci 4(4):568–578
Martinez-Moya P, Romero-Calvo I, Requena P, Hernandez-Chirlaque C, Aranda CJ, Gonzalez R, Zarzuelo A, Suarez MD, Martinez-Augustin O, Marin JJ, de Medina FS (2013) Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int Immunopharmacol 15(2):372–380
van den Buuse M, de Jong W (1989) Differential effects of dopaminergic drugs on open-field behavior of spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Pharmacol Exp Ther 248(3):1189–1196
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25(4):402–408
Hayat M (1986) Basic techniques for transmission electron microscopy, 1st edn. Academic Press, Orlando
Chun HS, Low WC (2012) Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells. Toxicology 292(2–3):105–112
Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC (2000) Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J 345(Pt 2):271–278
Jiang X, Jiang H, Shen Z, Wang X (2014) Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc Natl Acad Sci U S A 111(41):14782–14787
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122(Pt 4):437–441
Denault JB, Salvesen GS (2002) Caspases: keys in the ignition of cell death. Chem Rev 102(12):4489–4500
Thao TD, Ryu HC, Yoo SH, Rhee DK (2008) Antibacterial and anti-atrophic effects of a highly soluble, acid stable UDCA formula in Helicobacter pylori-induced gastritis. Biochem Pharmacol 75(11):2135–2146
Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M (2007) Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology 133(5):1603–1613
Rodriguez-Ortigosa CM, Banales JM, Olivas I, Uriarte I, Marin JJ, Corrales FJ, Medina JF, Prieto J (2010) Biliary secretion of S-nitrosoglutathione is involved in the hypercholeresis induced by ursodeoxycholic acid in the normal rat. Hepatology 52(2):667–677
Nathanson MH, Burgstahler AD, Masyuk A, Larusso NF (2001) Stimulation of ATP secretion in the liver by therapeutic bile acids. Biochem J 358(Pt 1):1–5
Lin SH (1989) Localization of the ecto-ATPase (ecto-nucleotidase) in the rat hepatocyte plasma membrane. Implications for the functions of the ecto-ATPase. J Biol Chem 264(24):14403–14407
Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB (1995) CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81(7):1063–1073
Isenberg JS, Klaunig JE (2000) Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci 53(2):340–351
Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT (2005) Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280(51):42026–42035
Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K (2002) Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol 17(2):196–202
Ishizaki K, Iwaki T, Kinoshita S, Koyama M, Fukunari A, Tanaka H, Tsurufuji M, Sakata K, Maeda Y, Imada T, Chiba K (2008) Ursodeoxycholic acid protects concanavalin A-induced mouse liver injury through inhibition of intrahepatic tumor necrosis factor-alpha and macrophage inflammatory protein-2 production. Eur J Pharmacol 578(1):57–64
Buryova H, Chalupsky K, Zbodakova O, Kanchev I, Jirouskova M, Gregor M, Sedlacek R (2013) Liver protective effect of ursodeoxycholic acid includes regulation of ADAM17 activity. BMC Gastroenterol 13(1):155
Rastogi RP, Sinha RP (2009) Apoptosis: molecular mechanisms and pathogenicity. EXCLI J 8:155–181
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4):481–490
Azzaroli F, Mehal W, Soroka CJ, Wang L, Lee J, Crispe IN, Boyer JL (2002) Ursodeoxycholic acid diminishes Fas-ligand-induced apoptosis in mouse hepatocytes. Hepatology 36(1):49–54
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z (2004) Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 79(1):137–146
Shah SA, Volkov Y, Arfin Q, Abdel-Latif MM, Kelleher D (2006) Ursodeoxycholic acid inhibits interleukin 1 beta [corrected] and deoxycholic acid-induced activation of NF-kappaB and AP-1 in human colon cancer cells. Int J Cancer 118(3):532–539
Joo SS, Won TJ, Lee DI (2004) Potential role of ursodeoxycholic acid in suppression of nuclear factor kappa B in microglial cell line (BV-2). Arch Pharm Res 27(9):954–960
Green DR, Beere HM (2000) Apoptosis. Gone but not forgotten. Nature 405(6782):28–29
Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G (1993) Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med 178(6):2207–2211
Miyaguchi S, Mori M (2005) Ursodeoxycholic acid (UDCA) suppresses liver interleukin 2 mRNA in the cholangitis model. Hepatogastroenterology 52(62):596–602
Keene CD, Rodrigues CM, Eich T, Linehan-Stieers C, Abt A, Kren BT, Steer CJ, Low WC (2001) A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington’s disease. Exp Neurol 171(2):351–360
Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ (2003) Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A 100(10):6087–6092
Rodrigues CM, Spellman SR, Sola S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ (2002) Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab 22(4):463–471
Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM (2008) Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med 14(2):54–62
Duan WM, Rodrigues CM, Zhao LR, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson’s disease. Cell Transplant 11(3):195–205
Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A 99(16):10671–10676
Castro RE, Sola S, Ramalho RM, Steer CJ, Rodrigues CM (2004) The bile acid tauroursodeoxycholic acid modulates phosphorylation and translocation of bad via phosphatidylinositol 3-kinase in glutamate-induced apoptosis of rat cortical neurons. J Pharmacol Exp Ther 311(2):845–852
Sola S, Castro RE, Laires PA, Steer CJ, Rodrigues CM (2003) Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol Med 9(9–12):226–234
Acknowledgments
The authors are grateful to Dr. Manal I. Salman, professor of Pathology, Faculty of Medicine, Ain Shams University, and vice director of Electron Microscopy Unit at Ain Shams University Specialized Hospital, for her efforts in the electron microscopic examinations.
Conflict of Interest
The authors declare that there are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelkader, N.F., Safar, M.M. & Salem, H.A. Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations. Mol Neurobiol 53, 810–817 (2016). https://doi.org/10.1007/s12035-014-9043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9043-8