Abstract
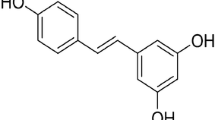
Excess production of reactive oxygen species in the brain has been implicated as a common underlying risk factor for the pathogenesis of a number of neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), and stroke. In recent years, there is considerable interest concerning investigation of antioxidative and anti-inflammatory effects of phenolic compounds from different botanical sources. In this review, we first describe oxidative mechanisms associated with stroke, AD, and PD, and subsequently, we place emphasis on recent studies implicating neuroprotective effects of resveratrol, a polyphenolic compound derived from grapes and red wine. These studies show that the beneficial effects of resveratrol are not only limited to its antioxidant and anti-inflammatory action but also include activation of sirtuin 1 (SIRT1) and vitagenes, which can prevent the deleterious effects triggered by oxidative stress. In fact, SIRT1 activation by resveratrol is gaining importance in the development of innovative treatment strategies for stroke and other neurodegenerative disorders. The goal here is to provide a better understanding of the mode of action of resveratrol and its possible use as a potential therapeutic agent to ameliorate stroke damage as well as other age-related neurodegenerative disorders.

Similar content being viewed by others
References
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Harman AW, Maxwell MJ (1995) An evaluation of the role of calcium in cell injury. Annu Rev Pharmacol Toxicol 35:129–144
Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G (1998) The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol [B] 168:149–158
Sun AY, Wang Q, Simonyi A, Sun GY (2008) Botanical phenolics and brain health. Neuromolecular Med 10:259–274
Farooqui T, Farooqui AA (2009) Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev 130:203–215
Rice-Evans C, Miller N (1997) Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukot Essent Fatty Acids 57:499–505
Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R (2002) Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol 135:1579–1587
Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB (2005) Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. Faseb J 19:455–457
Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G (2009) The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem 110:1445–1456
Sinclair DA (2005) Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126:987–1002
Allard JS, Perez E, Zou S, de Cabo R (2009) Dietary activators of Sirt1. Mol Cell Endocrinol 299:58–63
Anderson R, Prolla T (2009) PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta 1790:1059–1066
Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z (2009) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297:H13–H20
Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT (2009) Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci 14:376–397
Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E (2008) Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res 33:2444–2471
Traystman RJ (2003) Animal models of focal and global cerebral ischemia. ILAR J 44:85–95
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333
Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM (2003) Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology 61:1273–1275
Youdim KA, Joseph JA (2001) A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med 30:583–594
Deschamps V, Barberger-Gateau P, Peuchant E, Orgogozo JM (2001) Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemiology 20:7–15
Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY (2005) Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31:135–147
Curin Y, Ritz MF, Andriantsitohaina R (2006) Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem 4:277–288
Selkoe DJ, Podlisny MB (2002) Deciphering the genetic basis of Alzheimer's disease. Annu Rev Genom Hum Genet 3:67–99
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120:545–555
Selkoe DJ (2001) Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis 3:75–80
Reddy PH, Beal MF (2005) Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev 49:618–632
de la Monte SM, Wands JR (2005) Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis 7:45–61
Qiu WQ, Folstein MF (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging 27:190–198
Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS (2005) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120:701–713
Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB (2005) Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis 8:283–287
Selkoe DJ (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140:627–638
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298
Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY (2008) Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem 106:45–55
Butterfield DA, Reed T, Newman SF, Sultana R (2007) Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med 43:658–677
Langston JW (1987) MPTP: insights into the etiology of Parkinson's disease. Eur Neurol 26(Suppl 1):2–10
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Sun AY, Yang WL, Kim HD (1993) Free radical and lipid peroxidation in manganese-induced neuronal cell injury. Ann N Y Acad Sci 679:358–363
Koshimura I, Imai H, Hidano T, Endo K, Mochizuki H, Kondo T, Mizuno Y (1997) Dimethoxyphenylethylamine and tetrahydropapaverine are toxic to the nigrostriatal system. Brain Res 773:108–116
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res 34:55–65
Miller RL, Sun GY, Sun AY (2007) Cytotoxicity of paraquat in microglial cells: involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res 1167:129–139
Oldfield FF, Cowan DL, Sun AY (1991) The involvement of ethanol in the free radical reaction of 6-hydroxydopamine. Neurochem Res 16:83–87
Sun AY, Chen YM, James-Kracke M, Wixom P, Cheng Y (1997) Ethanol-induced cell death by lipid peroxidation in PC12 cells. Neurochem Res 22:1187–1192
Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K (2009) Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol 44:372–381
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36:357–368
Simonyi A, Zhang JP, Sun AY, Sun GY (1996) Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. NeuroReport 7:2115–2118
Simonyi A, Woods D, Sun AY, Sun GY (2002) Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res 26:352–357
Crews FT, Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44:115–127
Lev N, Ickowicz D, Barhum Y, Melamed E, Offen D (2009) DJ-1 changes in G93A-SOD1 transgenic mice: implications for oxidative stress in ALS. J Mol Neurosci 38:94–102
Muyderman H, Hutson PG, Matusica D, Rogers ML, Rush RA (2009) The human G93A-superoxide dismutase-1 mutation, mitochondrial glutathione and apoptotic cell death. Neurochem Res 34:1847–1856
Lunn JS, Hefferan MP, Marsala M, Feldman EL (2009) Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors 27:133–140
Zagami CJ, Beart PM, Wallis N, Nagley P, O'Shea RD (2009) Oxidative and excitotoxic insults exert differential effects on spinal motoneurons and astrocytic glutamate transporters: implications for the role of astrogliosis in amyotrophic lateral sclerosis. Glia 57:119–135
Gil JM, Rego AC (2008) Mechanisms of neurodegeneration in Huntington's disease. Eur J NeuroSci 27:2803–2820
Sun AY, Simonyi A, Sun GY (2002) The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med 32:314–318
Sun AY, Sun GY (2001) Ethanol and oxidative mechanisms in the brain. J Biomed Sci 8:37–43
Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S (2002) A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging 23:719–735
Chanvitayapongs S, Draczynska-Lusiak B, Sun AY (1997) Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. NeuroReport 8:1499–1502
Sun GY, Xia J, Draczynska-Lusiak B, Simonyi A, Sun AY (1999) Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. NeuroReport 10:93–96
Sun GY, Xia J, Xu J, Allenbrand B, Simonyi A, Rudeen PK, Sun AY (1999) Dietary supplementation of grape polyphenols to rats ameliorates chronic ethanol-induced changes in hepatic morphology without altering changes in hepatic lipids. J Nutr 129:1814–1819
Collins MA, Zou JY, Neafsey EJ (1998) Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J 12:221–230
Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J (2006) Cytokines and alcohol. Alcohol Clin Exp Res 30:720–730
Ranney A, Petro MS (2009) Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav Pharmacol 20:330–336
Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY (2002) Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 958:439–447
Kriz J, Lalancette-Hebert M (2009) Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol 117:497–509
Wang Q, Simonyi A, Li W, Sisk BA, Miller RL, Macdonald RS, Lubahn DE, Sun GY, Sun AY (2005) Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res 49:443–451
Wang Q, Sun AY, Simonyi A, Miller DK, Smith RE, Luchtefeld RG, Korthuis RJ, Sun GY (2009) Oral administration of grape polyphenol extract ameliorates cerebral ischemia/reperfusion-induced neuronal damage and behavioral deficits in gerbils: comparison of pre- and post-ischemic administration. J Nutr Biochem 20:369–377
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev 5:493–506
Sharma M, Gupta YK (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489–2498
Ono K, Naiki H, Yamada M (2006) The development of preventives and therapeutics for Alzheimer's disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des 12:4357–4375
Ono K, Yamada M (2006) Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem 97:105–115
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem 280:37377–37382
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179
Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM (2006) Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. Faseb J 20:2313–2320
Lu KT, Ko MC, Chen BY, Huang JC, Hsieh CW, Lee MC, Chiou RY, Wung BS, Peng CH, Yang YL (2008) Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem 56:6910–6913
Jin F, Wu Q, Lu YF, Gong QH, Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur J Pharmacol 600:78–82
Barber SC, Higginbottom A, Mead RJ, Barber S, Shaw PJ (2009) An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic Biol Med 46:1127–1138
Kumar P, Padi SS, Naidu PS, Kumar A (2006) Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol 17:485–492
Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S (2007) Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 294:137–144
Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B (2007) Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett 420:133–137
Rasouri S, Lagouge M, Auwerx J (2007) SIRT1/PGC-1: a neuroprotective axis? Med Sci (Paris) 23:840–844
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Lamming DW, Wood JG, Sinclair DA (2004) Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol 53:1003–1009
Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430:686–689
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Dasgupta B, Milbrandt J (2007) Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 104:7217–7222
Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A (2009) Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res 6:70–81
Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M (2007) Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem 282:37006–37015
Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE (2005) Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 130:685–696
Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN (2009) Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation 119:1124–1134
Rubiolo JA, Mithieux G, Vega FV (2008) Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol 591:66–72
Tang BL, Chua CE (2008) SIRT1 and neuronal diseases. Mol Aspects Med 29:187–200
Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY (2004) Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem 90:637–645
Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF (2005) Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. International immunopharmacology 5:185–193
Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH (2006) Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med 17:1069–1075
Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA (2008) Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun 372:254–259
Chen CY, Jang JH, Li MH, Surh YJ (2005) Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun 331:993–1000
Anekonda TS (2006) Resveratrol—a boon for treating Alzheimer's disease? Brain Res Rev 52:316–326
Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V (2007) Natural antioxidants in Alzheimer's disease. Expert Opin Investig Drugs 16:1921–1931
de la Lastra CA, Villegas I (2007) Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans 35:1156–1160
Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, Li XZ, Fang JG, Zhou B (2009) Antioxidant-based lead discovery for cancer chemoprevention: the case of resveratrol. J Med Chem 52:1963–1974
Saiko P, Szakmary A, Jaeger W, Szekeres T (2008) Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 658:68–94
Conte A, Pellegrini S, Tagliazucchi D (2003) Effect of resveratrol and catechin on PC12 tyrosine kinase activities and their synergistic protection from beta-amyloid toxicity. Drugs Exp Clin Res 29:243–255
Conte A, Pellegrini S, Tagliazucchi D (2003) Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Res Bull 62:29–38
Acknowledgement
This work was supported in part by NIH grant 2P01 AG018357.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, A.Y., Wang, Q., Simonyi, A. et al. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol Neurobiol 41, 375–383 (2010). https://doi.org/10.1007/s12035-010-8111-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-010-8111-y