Abstract
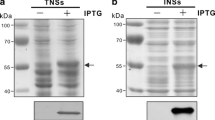
Partial recombinant secA proteins were produced from six different phytoplasma isolates representing five 16Sr groups and the expressed, purified recombinant (partial secA) protein from Cape St. Paul wilt disease phytoplasma (CSPWD, 16SrXXII) was used to immunise mice. Monoclonal antibodies (mAbs) were selected by screening hybridoma supernatants for binding to the recombinant proteins. To characterise the binding to proteins from different phytoplasmas, the antibodies were screened by ELISA and western blotting, and epitope mapping was undertaken. Eight different mAbs with varying degrees of specificity against recombinant proteins from different phytoplasma groups were selected. Western blotting revealed that the mAbs bind to proteins in infected plant material, two of which were specific for phytoplasmas. ELISA testing of infected material, however, gave negative results suggesting that either secA was not expressed at sufficiently high levels, or conformational changes of the reagents adversely affected detection. This work has shown that the phytoplasma secA gene is not a suitable antibody target for routine detection, but has illustrated proof of principle for the methodology.




Similar content being viewed by others
References
Lee, I.-M., Gundersen-Rindal, D. E., & Bertaccini, A. (1998). Phytoplasma: ecology and genomic diversity. Phytopathology, 88, 1359–1366.
IRPCM Phytoplasma/Spiroplasma Working Team—Phytoplasma taxonomy group. (2004). ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. International Journal of Systematic and Evolutionary Microbiology, 54, 1243–1255.
Firrao, G., Gibb, K., & Streten, C. (2005). Short taxonomic guide to the genus ‘Candidatus phytoplasma’. Journal of Plant Pathology, 87, 249–263.
Schneider, B., Gibb, K. S., & Seemüller, E. (1997). Sequence and RFLP analysis of the elongation factor Tu gene used in differentiation and classification of phytoplasmas. Microbiology, 143, 3381–3389.
Hodgetts, J., Boonham, N., Mumford, R., Harrison, N., & Dickinson, M. (2008). Phytoplasma phylogenetics based on analysis of the secA and 23S rRNA gene sequences for improved resolution of candidate species of ‘Candidatus Phytoplasma’. International Journal of Systematic and Evolutionary Microbiology, 58, 1826–1837.
Lee, I.-M., Zhao, Y., & Bottner, K. D. (2006). SecY gene sequence analysis for finer differentiation of diverse strains in the aster yellows phytoplasma group. Molecular and Cellular Probes, 20, 87–91.
Lee, I.-M., Gundersen-Rindal, D. E., Davis, R. E., & Bartoszyk, I. M. (1998). Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. International Journal of Systematic Bacteriology, 48, 1153–1169.
Hodgetts, J., Ball, T., Boonham, N., Mumford, R., & Dickinson, M. (2007). Use of terminal restriction fragment length polymorphism (T-RFLP) for identification of phytoplasmas in plants. Plant Pathology, 56, 357–365.
Nicolaisen, M., & Bertaccini, A. (2007). An oligonucleotide microarray-based assay for identification of phytoplasma 16S ribosomal groups. Plant Pathology, 56, 332–336.
Christensen, N. M., Nicolaisen, M., Hansen, M., & Schulz, A. (2004). Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Molecular Plant-Microbe Interactions, 17, 1175–1184.
Hodgetts, J., Boonham, N., Mumford, R., & Dickinson, M. (2009). Panel of 23S rRNA gene-based real-time PCR assays for improved universal and group-specific detection of phytoplasmas. Applied and Environment Microbiology, 75, 2945–2950.
Tomlinson, J. A., Boonham, N., & Dickinson, M. (2010). Development and evaluation of a one-hour DNA extraction and loop-mediated isothermal amplification assay for rapid detection of phytoplasmas. Plant Pathology, 59, 465–471.
Bekele, B., Hodgetts, J., Tomlinson, J., Boonham, N., Nikolic´, P., Swarbrick, P., et al. (2011). Use of a real-time LAMP isothermal assay for detecting 16SrII and XII phytoplasmas in fruit and weeds of the Ethiopian Rift Valley. Plant Pathology, 60, 345–355.
Hodgetts, J., Tomlinson, J., Boonham, N., González-Martín, I., Nikolić, P., Swarbrick, P., et al. (2011). Development of rapid in-field loop-mediated isothermal amplification (LAMP) assays for phytoplasmas. Bulletin of Insectology, 64, S41–S42.
Wei, W., Kakizawa, S., Jung, H.-Y., Suzuki, S., Tanaka, M., Nishigawa, H., et al. (2004). An antibody against the SecA membrane protein of one phytoplasma reacts with those of phylogenetically different phytoplasmas. Phytopathology, 94, 683–686.
Kakizawa, S., Oshima, K., Kuboyama, T., Nishigawa, H., Jung, H.-Y., Sawayanagi, T., et al. (2001). Cloning and expression analysis of Phytoplasma protein translocation genes. Molecular Plant Microbe Interaction, 14, 1043–1050.
Economou, A. (1999). Following the leader: Bacterial protein export through the Sec pathway. Trends in Microbiology, 7, 315–319.
Kakizawa, S., Oshima, K., Nishigawa, H., Jung, H.-Y., Wei, W., Suzuki, S., et al. (2004). Secretion of immunodominant membrane protein from onion yellows phytoplasma through the Sec protein-translocation system in Escherichia coli. Microbiology, 150, 135–142.
Berg, M., Davies, D. L., Clark, M. F., Vetten, H. J., Maie, G., Marcone, C., et al. (1999). Isolation of the gene encoding an immunodominant membrane protein of the apple proliferation phytoplamsa, and expression and characterization of the gene product. Microbiology, 145, 1937–1943.
Siampour, M., Izadpanah, K., Galetto, L., Salehi, M. C., & Marzachí, C. (2013). Molecular characterization, phylogenetic comparison and serological relationship of the Imp protein of several ‘Candidatus Phytoplasma aurantifolia’ strains. Plant Pathology, 62, 452–459.
Blomquist, C. L., Barbara, D. J., Davies, D. L., Clark, M. F., & Kirkpatrick, B. C. (2001). An immunodominant membrane protein gene from the Western X-disease phytoplasma is distinct from those of other phytoplasmas. Microbiology, 147, 571–580.
Boonham, N., Glover, R., Tomlinson, J., & Mumford, R. (2008). Exploiting generic platform technologies for the detection and identification of plant pathogens. European Journal of Plant Pathology, 121, 355–363.
Tsuda, S., Kameya-Iwaki, M., Hanada, K., Kouda, Y., Hikata, M., & Tomaru, K. (1992). A novel detection and identification technique for plant viruses: Rapid immunofilter paper assay (RIPA). Plant Disease, 76, 466–469.
Danks, C., & Barker, I. (2000). On-site detection of plant pathogens using lateral-flow devices. Bulletin of OEPP, 30, 421–426.
Braun-Kiewnick, A., Altenbach, D., Oberhänsli, T., Bitterlin, W., & Duffy, B. (2011). A rapid lateral-flow immunoassay for phytosanitary detection of Erwinia amylovora and on-site fire blight diagnosis. Journal of Microbiol Methods, 87, 1–9.
Thornton, C. R., Groenhof, A. C., Forrest, R., & Lamotte, R. (2004). A one-step, immunochromatographic lateral flow device specific to Rhizoctonia solani and certain related species, and its use to detect and quantify R. solani in soil. Phytopathology, 94, 280–288.
Lane, C. R., Hobden, E., Walker, L., Barton, V. C., Inman, A. J., Hughes, K. J. D., et al. (2007). Evaluation of a rapid diagnostic field test kit for identification of Phytophthora species, including P. ramorum and P. kernoviae at the point of inspection. Plant Pathology, 56, 828–835.
De Boer, S. H., & López, M. M. (2012). New grower-friendly methods for plant pathogen monitoring. Annual Review of Phytopathology, 50, 197–218.
Harlow, E., & Lane, D. (1988). Antibodies: A laboratory manual. New York: Cold Spring Harbour Laboratory Press.
Sambrook, J., & Russell, D. (2000). Molecular cloning: A laboratory manual. New York: Cold Spring Harbour Laboratory Press.
Barbara, D. J., Morton, A., Clark, M. F., & Davies, D. L. (2002). Immunodominant membrane proteins from two phytoplasmas in the aster yellows clade (chlorante aster yellows and clover phyllody) are highly divergent in the major hydrophilic region. Microbiology, 148, 157–167.
Hu, J. S., Li, H. P., Barry, K., & Wang, M. (1995). Comparison of dot blot, ELISA, and RT-PCR assays for detection of two cucumber mosaic virus isolates infecting banana in Hawaii. Plant Disease, 79, 902–906.
Olmos, A., Dasí, M. A., Candresse, T., & Cambra, M. (1996). Print-capture PCR: a simple and highly sensitive method for the detection of Plum pox virus (PPV) in plant tissues. Nucleic Acids Research, 24, 2192–2193.
Hogenhout, S. A., Oshima, K., Ammar, el-D, Kakizawa, S., Kingdom, H. N., & Namba, S. (2008). Phytoplasmas: Bacteria that manipulate plants and insects. Molecular Plant Pathology, 9, 403–423.
Massumi, H., Jones, P., & Hague, N. (2005). Partial epitope mapping of Alfalfa mosaic virus and the effect of coat protein gene mutation on aphid transmission. Iranian Journal of Biotechnology, 3, 31–40.
Acknowledgments
This work was performed as part of a Defra Plant Health Division-funded Taxonomic Fellowship (for JH). The authors would like to thank Professor Phil Jones (Rothamsted Research, UK), Dr. Jaroslava Přibylova (Institute of Plant Molecular Biology, Czech Republic), Dr. Joseph Nipah (CSIR, Sekondi, Ghana), Dr. Nigel Harrison (University of Florida, USA), and Professor Assunta Bertaccini (University of Bologna, Italy) for providing samples. Phytoplasmas were held under Defra Plant Health Licence No. PHL 173B/5244.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hodgetts, J., Johnson, G., Perkins, K. et al. The Development of Monoclonal Antibodies to the secA Protein of Cape St. Paul Wilt Disease Phytoplasma and Their Evaluation as a Diagnostic Tool. Mol Biotechnol 56, 803–813 (2014). https://doi.org/10.1007/s12033-014-9759-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9759-8