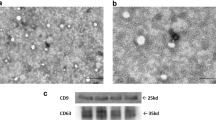
Abstract
Mesenchymal stem cell (MSC) therapy is a promising prospect for the treatment of Alzheimer’s disease (AD); however, the underlying mechanisms by which MSCs mediate positive effects are still unclear. We speculated that MSCs mediate microglial autophagy and enhance the clearance of Aβ. To test this hypothesis, we cultured BV2 microglial cells with umbilical cord mesenchymal stem cells conditioned medium (ucMSCs-CM) in the presence or absence of Aβ25–35 oligomers. We investigated BV2 cell proliferation, cell death, and Aβ25–35 phagocytosis as well as protein expression levels of LC3, Beclin-1, p62, insulin-degrading enzyme (IDE), and neprilysin (Nep) with western blotting. The results showed that ucMSCs-CM inhibited the proliferation and decreased cell death of BV2 cells induced by Aβ25–35. ucMSCs-CM also promoted the phagocytosis of Aβ25–35 by BV2 cells and changed the expression of autophagy-related proteins LC3, Beclin-1, and p62. Treatment also upregulated the expression of Aβ-degrading enzymes IDE and Nep. Furthermore, the culture medium in BV2 cells with Aβ25–35 and ucMSCs-CM prevented neuronal cell SH-SY5Y from cell death compared to control medium without ucMSCs-CM. Altogether, these data suggested that ucMSCs-CM protect microglial and neuronal cells from Aβ25–35-induced cell death and promote Aβ phagocytosis by modulating autophagy and enhancing the expression of Aβ-degrading enzymes in microglia.







Similar content being viewed by others
References
Alzheimer's Disease International (2016) World Alzheimer Report. Alzheimer's Disease International, London
Baik SH, Kang S, Son SM, Mook-Jung I (2016) Microglia contributes to plaque growth by cell death due to uptake of amyloid beta in the brain of Alzheimer's disease mouse model. Glia 64(12):2274–2290
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci 28(27):6926–6937
Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noel A, Brook G, Schoenen J, Franzen R (2013) Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One 8(8):e69515
Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, Kim HM, Kim DH, Yoon SY (2014) Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10(10):1761–1775
Ciregia F, Urbani A, Palmisano G (2017) Extracellular vesicles in brain tumors and neurodegenerative diseases. Front Mol Neurosci 10:276
Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA (2018) Dysfunction of autophagy and endosomal–lysosomal pathways: roles in pathogenesis of down syndrome and Alzheimer's disease. Free Radic Biol Med 114:40–51
Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, Lv P, Xing Q, Qu R, Yao N, Yang B, Guan F (2017) Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer's disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res 320:291–301
Dagher NN, Najafi AR, Kayala KMN, Elmore MRP, White TE, Medeiros R, West BL, Green KN (2015) Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation 12:139
De Strooper B, Karran E (2016) The cellular phase of Alzheimer's disease. Cell 164(4):603–615
Dowling P, Clynes M (2011) Conditioned media from cell lines: a complementary model to clinical specimens for the discovery of disease-specific biomarkers. Proteomics 11(4):794–804
Ehrhart J, Darlington D, Kuzmin-Nichols N, Sanberg CD, Sawmiller DR, Sanberg PR, Tan J (2016) Biodistribution of infused human umbilical cord blood cells in Alzheimer's disease-like murine model. Cell Transplant 25(1):195–199
Fan Z, Brooks DJ, Okello A, Edison P (2017) An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain 140(3):792–803
Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, El Khoury J (2013) Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer's-like disease progression. Nat Commun 4:2030
Frere S, Slutsky I (2018) Alzheimer's disease: from firing instability to homeostasis network collapse. Neuron 97(1):32–58
Giulian D, Baker TJ (1986) Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6(8):2163–2178
Godoy MA, Saraiva LM, Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva LRP, Leal RB, Monteiro VHS, Braga CV, Araujo-Silva CA, Sinis LC, Santos VB, Brunswick THK, Alcantara CL, Lima A, Cunha ESNLD, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST (2017) Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem 293(6):1957–1975
Guglielmotto M, Monteleone D, Piras A, Valsecchi V, Tropiano M, Ariano S, Fornaro M, Vercelli A, Puyal J, Arancio O, Tabaton M, Tamagno E (2014) Aβ1–42 monomers or oligomers have different effects on autophagy and apoptosis. Autophagy 10(10):1827–1843
Hamisha KN, Tfilin M, Yanai J, Turgeman G (2015) Mesenchymal stem cells can prevent alterations in behavior and neurogenesis induced by Ass25-35 administration. J Molec Neurosci 55(4):1006–1013
Hickman SE, Allison EK, Khoury JE (2008) Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28(33):8354–8360
Huang F-L, Shiao Y-J, Hou S-J, Yang C-N, Chen Y-J, Lin C-H, Shie F-S, Tsay H-J (2013) Cysteine-rich domain of scavenger receptor AI modulates the efficacy of surface targeting and mediates oligomeric Aβ internalization. J Biomed Sci 20(1):54
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6):1204–1222
Hunsberger JG, Rao M, Kurtzberg J, Bulte JWM, Atala A, LaFerla FM, Greely HT, Sawa A, Gandy S, Schneider LS, Doraiswamy PM (2016) Accelerating stem cell trials for Alzheimer's disease. Lancet Neurol 15(2):219–230
Jang SK, Yu JM, Kim ST, Kim GH, Park DW, Lee DI, Joo SS (2015) An Aβ42 uptake and degradation via Rg3 requires an activation of caveolin, clathrin and Aβ-degrading enzymes in microglia. Eur J Pharmacol 758:1–10
Janssen L, Dubbelaar ML, Holtman IR, de Boer-Bergsma J, Eggen BJ, Boddeke HW, De Deyn PP, Van Dam D (2017) Aging, microglia and cytoskeletal regulation are key factors in the pathological evolution of the APP23 mouse model for Alzheimer's disease. Biochim Biophys Acta 1863(2):395–405
Jha NK, Jha SK, Kumar D, Kejriwal N, Sharma R, Ambasta RK, Kumar P (2015) Impact of insulin degrading enzyme and neprilysin in Alzheimer's disease biology: characterization of putative cognates for therapeutic applications. J Alzheimers Dis 48(4):891–917
Jiang P, Mizushima N (2015) LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75:13–18
Khabbal J, Kerkela E, Mitkari B, Raki M, Nystedt J, Mikkonen V, Bergstrom K, Laitinen S, Korhonen M, Jolkkonen J (2015) Differential clearance of rat and human bone marrow-derived mesenchymal stem cells from the brain after intra-arterial infusion in rats. Cell Transplant 24(5):819–828
Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, Na DL, Oh W, Chang JW (2015) GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer's disease model. Stem Cells Dev 24(20):2378–2390
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW (2012) Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-beta plaques. Cell Death Differ 19(4):680–691
Kumar K, Kumar A, Keegan RM, Deshmukh R (2017) Recent advances in the neurobiology and neuropharmacology of Alzheimer's disease. Biomed Pharmacother 98: 297–307
Lane CA, Hardy J, Schott JM (2018) Alzheimer's disease. Eur J Neurol 25(1):59–70
Larsen KB, Lamark T, Overvatn A, Harneshaug I, Johansen T, Bjorkoy G (2010) A reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy 6(6):784–793
Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, Jin HK, Bae JS (2010) The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci Lett 481(1):30–35
Lee JH, Oh I-H, Lim HK (2016) Stem cell therapy: a prospective treatment for Alzheimer's disease. Psychiatry Investig 13(6):583–589
Li Q, Liu Y, Sun M (2017) Autophagy and Alzheimer's disease. Cell Mol Neurobiol 37(3):377–388
Li T, Xia M, Gao Y, Chen Y, Xu Y (2015) Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther 15(9):1293–1306
Mallard C, Tremblay ME, Vexler ZS (2018) Microglia and neonatal brain injury. Neuroscience Jan 17 [Epub ahead of print]
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330(6012):1774
Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Fullgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, Skidmore J, Rubinsztein DC (2017) Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93(5):1015–1034
Miners JS, Barua N, Kehoe PG, Gill S, Love S (2011) Abeta-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol 70(11):944–959
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140(3):313–326
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64(2):113–122
Orhon I, Reggiori F (2017) Assays to monitor autophagy progression in cell cultures. Cell 6(3):20
Plaza-Zabala A, Sierra-Torre V, Sierra A (2017) Autophagy and microglia: novel partners in neurodegeneration and aging. Int J Mol Sci 18(3):598
Quek C, Hill AF (2017) The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun 483(4):1178–1186
Ryan JM, Barry FP, Murphy JM, Mahon BP (2005) Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2:8
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH (2014) Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy 10(1):32–44
Sole-Domenech S, Cruz DL, Capetillo-Zarate E, Maxfield FR (2016) The endocytic pathway in microglia during health, aging and Alzheimer's disease. Ageing Res Rev 32:89–103
Spangenberg EE, Green KN (2017) Inflammation in Alzheimer's disease: lessons learned from microglia-depletion models. Brain Behav Immun 61:1–11
Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN (2016) Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139(Pt 4):1265–1281
Sun W, Xu Z, Liu R, Yuan Z, Feng H (2013) Comparison of different fetal bovine serums in cultivation of human umbilical cord stroma-derived mesenchymal stem cells. J Xinxiang Med Univ 30(10):787–789,793
Tejera D, Heneka MT (2016) Microglia in Alzheimer's disease: the good, the bad and the ugly. Curr Alzheimer Res 13(4):370–380
Waisman A, Ginhoux F, Greter M, Bruttger J (2015) Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol 36(10):625–636
Wang SS, Jia J, Wang Z (2018a) Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in Alzheimer's disease mice. J Alzheimers Dis 61(3):1005–1013
Wang X, Ma S, Yang B, Huang T, Meng N, Xu L, Xing Q, Zhang Y, Zhang K, Li Q, Zhang T, Wu J, Yang GL, Guan F, Wang J (2018b) Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer's disease. Behav Brain Res 339:297–304
Wes PD, Sayed FA, Bard F, Gan L (2016) Targeting microglia for the treatment of Alzheimer's disease. Glia 64(10):1710–1732
Whyte LS, Lau AA, Hemsley KM, Hopwood JJ, Sargeant TJ (2017) Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer's disease? J Neurochem 140(5):703–717
Wood H (2017) Alzheimer disease: soluble TREM2 in CSF sheds light on microglial activation in AD. Nat Rev Neurol 13(2):65
Wray S, Fox NC (2016) Stem cell therapy for Alzheimer's disease: hope or hype? Lancet Neurol 15(2):133–135
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain 134(Pt 1):258–277
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18(9):1865
Yu Y, Ye RD (2015) Microglial Abeta receptors in Alzheimer's disease. Cell Mol Neurobiol 35(1):71–83
Zuroff L, Daley D, Black KL, Koronyo-Hamaoui M (2017) Clearance of cerebral Abeta in Alzheimer's disease: reassessing the role of microglia and monocytes. Cell Molec Life Sci 74(12):2167–2201
Acknowledgements
We would like to thank Editage [www.editage.cn] for English language editing. This study was supported by the Doctoral Research Start-up Fund of Xinxiang Medical University, Scientific Research Fund of Xinxiang Medical University (2013QN122), Postdoctoral Research Fund of Henan Province (2013041), Project of Science and Technology Department of Henan Province (162102310493), Key Project of Science and Technology Research of Henan Provincial Education Department (14B180027), National Natural Science Foundation of China (131600791, 81771226), Xinxiang City Foundation (CXRC16003 and ZD17008), Xinxiang Medical University Foundation (20172DCG-03), and National Training Program of Innovation and Entrepreneurship for Undergraduates of China (201610472051).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure of Potential Conflicts of Interest
All authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Xu, Z., Nan, W., Zhang, X. et al. Umbilical Cord Mesenchymal Stem Cells Conditioned Medium Promotes Aβ25-35 phagocytosis by Modulating Autophagy and Aβ-Degrading Enzymes in BV2 Cells. J Mol Neurosci 65, 222–233 (2018). https://doi.org/10.1007/s12031-018-1075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1075-5