Abstract
Background
An external ventricular drain (EVD) is the gold standard for measurement of intracranial pressure (ICP) and allows for drainage of cerebrospinal fluid (CSF). Different causes of elevated ICP, such as CSF outflow obstruction or cerebral swelling, respond differently to CSF drainage. This is a widely recognized but seldom quantified distinction. We sought to define an index to characterize the response to CSF drainage in neurocritical care patients.
Methods
We studied consecutive patients admitted to the neurointensive care unit who had an EVD. The EVD was closed for 30 min prior to assessment. We documented pre-drainage ICP, opened EVD to drainage allowing CSF to drain until it ceased, and recorded post-drainage ICP at EVD closure. We calculated the pressure equalization (PE) ratio as the difference between pre-drainage ICP and post-drainage ICP divided by the difference between pre-drainage ICP and EVD height.
Results
We studied 60 patients (36 traumatic brain injury [TBI], 24 non-TBI). As expected, TBI patients had more signs of cerebral swelling on CT and smaller ventricles. Although TBI patients had significantly higher pre-drainage ICP (26 ± 10 mm Hg) than non-TBI patients (19 ± 5 mm Hg, p < 0.001) they drained less CSF (7 cc vs. 4 cc, p < 0.01). PE ratio was substantially higher in non-TBI than in TBI patients (0.86 ± 0.36 vs. 0.43 ± 0.31, p < 0.0001), indicating that non-TBI patients were better able to equalize pressure with EVD height than TBI patients.
Conclusions
PE ratio reflects the ability to equalize pressure with the preset height of the EVD and differs substantially between TBI and non-TBI patients. A high PE ratio likely indicates CSF outflow obstruction effectively treated by CSF diversion, while a lower PE ratio occurs when cerebral swelling predominates. Further studies could assess whether the PE ratio would be useful as a surrogate marker for cerebral edema or the state of intracranial compliance.
Similar content being viewed by others
Introduction
Measurement of the intracranial pressure (ICP) has been a cornerstone of monitoring patients with severe traumatic brain injury (TBI) and other critical brain pathologies since its introduction into clinical care [1,2,3,4]. The external ventricular drain (EVD) was the first method described to monitor ICP and remains the only method which both measures ICP and allows for the therapeutic drainage of cerebrospinal fluid (CSF) to decrease ICP [5,6,7].
In some clinical situations, EVDs are used to temporarily divert CSF when it cannot be absorbed. A common clinical scenario in which this occurs is as after spontaneous subarachnoid hemorrhage when CSF outflow obstruction leads to acute non-absorptive hydrocephalus. In this situation, CSF diversion via an external drain is a very efficient means of responding to the underlying problem of CSF outflow obstruction, so that the desired level of ICP is usually attained. In contrast, in patients with severe TBI elevated ICP is usually due to a combination of pathophysiological factors that include discrete hemorrhagic mass lesions, brain edema, and disturbed cerebral vasomotor reactivity—none of which can be resolved directly with diversion of the CSF. Therapeutic use of an EVD in severe TBI relies on an incremental benefit provided by movement along the pressure–volume curve within the cranium. The obvious difference between these two clinical scenarios has long been recognized and utilized in clinical practice. However, to date few efforts have been made to quantitatively characterize the varying patterns of response of the ICP to CSF drainage in different pathological states. We sought to develop a practical method to quantitatively describe the pressure response to CSF drainage in patients with an EVD and to assess differences in the pressure response between severe TBI patients and patients with other pathologies.
Methods
We studied consecutive patients admitted to the neurosurgical intensive unit at our institution that had an EVD placed as per clinical indication. EVD was placed on the side determined by clinical need. EVD height was set by the clinical indication and opened to drainage at a set height or at above a threshold ICP of 20 mm Hg per clinical indication. This study was approved by the Hadassah-Hebrew University Medical Center Institutional Review Board (IRB approval number 19384). Waiver of informed consent was approved for this study, as no deviation from standard care or therapeutic intervention was a part of this study.
In all patients, a CSF drainage challenge was performed to assess the ability of CSF drainage to equalize pressure with the height of the EVD. The challenge was performed after the EVD had been closed for 30 min prior to the challenge. Before the PE ratio was assessed, all patients were evaluated to assure adequate sedation. If any resistance to the ventilator, spontaneous motor movements, or other signs of wakefulness were present sedation was increased until these ceased. The CSF drainage challenge and assessment of pressure equalization were performed as follows:
The ICP immediately prior to opening of the EVD was recorded (ICPinitial). The drain height zeroed at the level of the external auditory meatus was also recorded (EVDheight). The drain was opened to allow drainage of CSF until CSF stopped draining. Immediately upon cessation of CSF drainage, the EVD was closed and the post-drainage ICP (ICPpost) was recorded. The difference between initial ICP and the post-drainage ICP measured immediately after EVD closure was calculated as follows:
In cases of pure obstruction to the outflow of CSF, end ICP after drainage (ICPpost) would be expected to equalize with the preset height of the EVD (EVDheight). Therefore, when elevated ICP results from pure outflow obstruction, the maximal expected decrease in ICP is defined as follows:
We defined the ratio between the actual and expected decrease in ICP as follows:
We termed this ratio of
the pressure equalization ratio, because it reflects the degree to which an equalization of pressure is achieved between the EVD height and the ICP after CSF is drained. When CSF drainage leads to a decrease in ICP to the level of drain height the pressure equalization ratio is 1. When no CSF is drained and ICP is unchanged from its initial value, the pressure equalization ratio is 0. The pressure equalization ratio will therefore generally vary between 0 and 1, reflecting the degree to which equalization is achieved between post-drainage ICP and the height of the EVD.
We evaluated the first computed tomography (CT) scan after EVD placement in all patients, calculating Rotterdam score [8] in TBI patients, and Fisher score [9] in spontaneous SAH patients. In intracerebral hemorrhage (ICH) patients, we calculated the ICH score [10] and recorded ICH location. In all patients, we assessed the presence and degree of midline shift and the presence of cisternal compression or complete effacement. In addition, we measured ventricular volume on CT scans to evaluate whether this parameter may influence CSF drainage characteristics. Ventricular volume was measured using a semi-automated program (IntelliSpace Portal, Philips Medical Systems, Best, The Netherlands).
Statistics
All continuous parameters are reported as mean and standard deviation. Non-continuous parameters are reported as median and interquartile range. An unpaired t test was used to compare continuous data. A χ2 test was used to compare nonparametric data.
Results
We studied 60 patients, 36 with TBI and 24 with other pathologies. Patient characteristics are detailed in Table 1. Male gender was more common, and median Glagow Coma Score (GCS) was one point lower in the TBI group, but the difference in GCS did not reach statistical significance. Mean intensive care unit (ICU) days and days with an EVD were not different between groups. In the non-TBI group, 88% of patients had spontaneous subarachnoid hemorrhage (SAH). In all of these patients, an aneurysm was diagnosed and all but one underwent endovascular treatment. One elderly patient with SAH presented in poor neurological state, did not undergo intervention, and died. Patients with hemorrhagic stroke constituted 12% of the non-TBI patients, and all underwent surgical intervention.
Post-EVD placement CT scan characteristics of TBI and non-TBI patients are detailed in Table 2. A greater percentage of patients with TBI patients had some degree of midline shift compared with non-TBI patients (45% vs. 25%, respectively). Likewise, a greater percentage of TBI patients had cisternal compression or complete effacement compared with non-TBI patients (56% vs. 25%, respectively), indicating that more TBI patients had signs of substantial brain edema on CT scans. As expected, ventricular volume was substantially smaller in TBI patients than non-TBI patients (Table 2), likely indicating a greater propensity to have a more edematous brain resulting in ventricular compression. CT scans of a patient with severe TBI and aneurysmal subarachnoid hemorrhage are presented in Fig. 1.
Representative CT scans of a traumatic brain injury (TBI) patient (a, b) and a patient with spontaneous subarachnoid hemorrhage secondary to a ruptured aneurysm (c, d). The CT scan of the TBI patient demonstrates bilateral temporal contusions with brain edema and compressed cisterns (a). As expected, the ventricles are small and an external ventricular drain (EVD) is in place (b). The CT scan of the patient with aneurysmal subarachnoid hemorrhage blood demonstrates blood in the basal cisterns (c) and large ventricles with EVD in place (d). Intracranial pressure (ICP) elevation in the TBI patient is more likely to result from the mass lesions and cerebral swelling leading to poor intracranial compliance, while in the patient with aneurysmal subarachnoid hemorrhage ICP elevation is likely to result from an impairment of cerebrospinal fluid (CSF) reabsorption that can be relieved with diversion of CSF
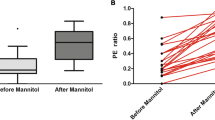
Physiological data and EVD drainage parameters at the time of CSF drainage challenge are presented in Table 3. We performed 50 measurements of CSF drainage parameters in 24 non-TBI patients and 70 measurements in 36 TBI patients. In TBI patients, drain height was set to a mean height of 7 ± 1 mm Hg, corresponding to a height of 10 cm above the external auditory meatus. In non-TBI patients, the EVD height was generally slightly higher (mean 11 ± 1 mm Hg) reflecting our clinical practice to drain at height of 15 cm above the zero point in these patients. Initial ICP prior to drainage was significantly higher in the TBI group than the in the non-TBI group (Table 3). With opening of the EVD, the mean volume of CSF drained in the TBI group was less than in the non-TBI group (Table 3). Post-drainage ICP remained higher in the TBI group than the non-TBI group (Table 3). Importantly, the pressure equalization ratio, was substantially lower in TBI patients (0.43 ± 0.31) compared to non-TBI patients (0.86 ± 0.36; p < 0.0001), indicating a markedly reduced propensity of CSF drainage to achieve a post-drainage ICP equal to EVD height in the TBI group (Fig. 2).
Pressure equalization (PE) ratio in TBI is substantially lower than in non-TBI patients, indicating that TBI patients are less likely to achieve a pressure near that of the external ventricular drain height following drainage of cerebrospinal fluid (CSF). The higher PE ratio in non-TBI patients indicates that these patients were better able to achieve an intracranial pressure nearer the EVD height, likely reflecting an underlying pathology of CSF outflow obstruction in these patients. In TBI patients, the lower PE ratio likely reflects a predominance of brain edema and inherently poor intracranial compliance, a situation in which the elevation in intracranial pressure can only be partially ameliorated by CSF drainage. *p < 0.0001
Discussion
In this study, we sought to define a quantitative parameter that describes the response to CSF drainage in patients with an EVD. The parameter we describe, the pressure equalization ratio, by its definition, reflects the ability of CSF drainage to achieve a pressure equal to the preset height of the EVD. This is important because in theory it can distinguish between those pathological states in which this occurs easily and those in which it does not. The assessment we performed may be conceptualized as a CSF drainage challenge that quantifies the response to CSF drainage in a standardized fashion. Not surprisingly, our results indicate that there are substantial differences in the pressure equalization ratio between patients with severe TBI and those with other pathologies. Non-TBI patients drained CSF in larger volumes and were able to achieve a higher pressure equalization ratio, indicating a better ability to equalize the ICP with the height of the EVD. In contrast, TBI patients drained less CSF despite having higher initial ICP and had a lower pressure equalization ratio, indicating that it was more difficult to achieve an ICP equal to EVD height following CSF drainage in these patients. The different responses most likely reflect fundamental differences in the intracranial pathology between these two groups, including differences in the intracranial compliance. Importantly, non-TBI patients had fewer signs of severe brain edema on CT scans and larger ventricles (Table 2). It is likely that in many of these patients the underlying pathology was not primarily brain edema, but rather an impairment of CSF reabsorption, so that they could be effectively treated with CSF diversion. Not surprisingly, severe TBI patients had substantially more signs of brain edema and smaller ventricles on CT scan, making it less likely that CSF drainage alone could return ICP to normal values. An edematous brain with small ventricles often represents a clinical scenario of poor intracranial compliance. While the varying patterns of CSF drainage may be intuitively obvious to practitioners of neurocritical care, we demonstrate a potentially useful method to quantify the differing responses to CSF drainage in patients with different brain pathologies that may be a correlate of the state of intracranial compliance. In a recent study, Lai and colleagues described a volume–pressure indicator, obtained by calculating the parabolic regression indicator they term “a,” to assess the decrease in ICP in response to CSF drainage to a pressure of 10 mm Hg [11]. They observed a substantial difference in the calculated parameter “a” between patients with hydrocephalus and those with mass lesion and edema, suggesting that the parabolic regression indicator reflects the intracranial volume–pressure relationship. Our results support these findings and present a simple index that may be advantageous to the clinician since it requires only a simple calculation that can be performed at bedside.
The reason patients with true CSF outflow obstruction will equalize pressure with the height of the EVD is clear. Outflow obstruction, either due to obstruction of the ventricular system itself, or due to problems with reabsorption of CSF at the level of the arachnoid granulations, can easily be treated with diversion of CSF flow into an external system. The free flow of CSF thus enabled will naturally equilibrate with the pressure at the external setting (drain height). In patients in whom the underlying pathology is not one of pure outflow obstruction of the CSF, the reasons for the lack of equilibration with the external system are less intuitively obvious. One possible explanation is that the ventricles are smaller in patients with TBI since the brain is often edematous after traumatic injury. Not surprisingly, our findings confirm that mean ventricular size is reduced after TBI (Table 2). However, actual ventricular collapse on CT scan was not common in our series, raising the question of why CSF drainage cannot continue beyond a certain threshold in TBI patients. In this respect, the difference in ventricular size between TBI and non-TBI patients provides only a partial explanation of why ICP fails to equalize with a preset external pressure in TBI patients.
Another possible explanation for the difficulty in equalizing pressures may lie in the interaction between cerebral cellular swelling, ventricular size, CSF flow, and bulk flow of the brain interstitial fluid (ISF). In severe TBI patients, the most common reason for elevated ICP is the presence of traumatic mass lesions and the subsequent brain edema that surround these lesions which often leads to ventricular compression. The traumatic mass lesions and the extra water content that result from brain edema elevate ICP in and of themselves due to the volume–pressure relationship in the closed cranium. But in addition, brain edema, and especially intracellular cerebral edema that occurs soon after TBI [12, 13], leads to a change in the relationship between the intracellular and extracellular compartments in the brain [14]. Cerebral intracellular swelling also occurs after stroke where it may be profound, decreasing the volume of the extracellular space by up to 50% [15,16,17]. These pathophysiological changes may influence the circulation of ISF within the extracellular space of the brain (ECSB). Recent experimental studies have found impaired flow of ISF through the brain following TBI [18]. Overall, the rate of ISF flow is substantially slower than the estimated rate of CSF flow [19, 20]. However, the overall volume of brain ISF is estimated to be from slightly greater to close to twice the volume of intracranial CSF [21,22,23], so although its flow occurs at a substantially slower rate its overall effect on ICP may still be significant. A recent review suggests that the CSF-ECSB may be conceptualized as one large compartment measuring about 300 cc in humans with the ECSB having many tortuous arms [21]. While classical landmark studies of CSF circulation that defined ICP in relation to resistance to CSF flow measured resistance to flow in terms of resistance to CSF outflow [24], resistance to ISF flow within the ECSB may also influence ICP. A disruption of the normal dynamics between ISF and CSF flow caused by an increased resistance to flow of ISF within the swollen brain parenchyma may lead to a pressure build up that compresses the ventricles and can only be partially relieved by CSF drainage. In these cases, ICP will not easily equilibrate with the pressure of an external drain. This may help account for the observed differences in PE ratio between TBI and non-TBI patients. The precise pathophysiological mechanisms that best account for this phenomenon will need to be investigated in future studies.
The parameter we describe, the pressure equalization ratio, is simple in that it consists only of recording information from the most widely used intracranial monitor, the EVD. Calculating the pressure equalization ratio is straightforward and can easily be done at bedside, potentially at frequent intervals. It may be a useful surrogate marker for the degree of cerebral swelling (as opposed to CSF outflow obstruction) and thus reflect the extent to which mass lesions and brain edema interfere with the normal interaction between ISF flow, CSF flow, and ventricular size in the injured brain. In a specialized neurointensive care unit, a careful reading of the patient bedside chart that indicates whether CSF drainage was effective or not in reducing ICP may give a qualitative assessment of the effectiveness of CSF drainage in reducing elevated ICP. However, such assessments cannot provide a quantitative assessment of the effectiveness of CSF drainage, nor can they provide a method to quantitatively assess differences between patients. Future studies, should also seek to compare PE ratio to other surrogate markers of the state of intracranial compliance such as ICP pulse pressure, the ratio of the P1 portion of the ICP wave to its P2 portion, and the correlation coefficient between the pulse amplitude of the ICP and ICP (RAP) [25, 26]. It is important to reiterate that although an equalization of pressure with the EVD height may not be achievable in TBI patients with severe brain edema, the incremental benefit of movement along the pressure volume curve achieved by CSF drainage is of great and obvious benefit in maintaining ICP below threshold values. In future studies, we intend to explore whether the pressure equalization ratio can be used to follow patients over time to assess changes in the degree of cerebral edema, to serve as an indicator for the need for hyperosmolar therapy, and to evaluate the efficacy of interventions to ameliorate cerebral swelling. In addition, we plan to measure PE ratio before and after secondary decompressive craniectomy for intractably elevated ICP in order to directly assess whether PE ratio reflects the state of intracranial compliance. Only by measuring PE ratio in different clinical contexts will it be possible to determine its usefulness as one of the indices that may help to evaluate patients after severe brain injury.
Limitations
Our study has several limitations. First, in this preliminary investigation we only assessed differences between TBI and non-TBI patients. We did not evaluate changes in the pressure equalization ratio over the ICU course to determine if this parameter can be used as a marker of worsening pathology or response to therapy in individual patients. This is a goal of future studies. Second, we did not measure the rate of CSF drainage over a high-resolution time course to determine if the precise rate of CSF drainage can also be used to characterize differences between patients and the response to therapeutic interventions. In future studies, we intend to assess whether high-frequency measurements of the response to a CSF drainage challenge obtained while drainage is ongoing provide added value in comparison with a discrete measure of the PE ratio. Lastly, in this observational study, we did not have the resources to perform imaging techniques that would allow us to compare the observed patterns of CSF drainage with evaluations of water content on magnetic resonance imaging in order to better characterize the relationship between CSF drainage parameters and cerebral edema.
Conclusions
We describe a parameter, the pressure equalization ratio, to characterize the response to CSF drainage from an EVD. In this preliminary study, we demonstrate a quantifiable difference in the pressure equalization ratio between TBI and non-TBI patients that may be related to underlying differences in pathophysiology. Further studies are needed to evaluate whether the pressure equalization ratio can serve as clinically useful surrogate marker of the degree of cerebral edema or the state of intracranial compliance, and as a means to assess the response to therapeutic interventions to decrease brain swelling.
References
Guillaume J, Janny P. Continuous intracranial manometry; physiopathologic and clinical significance of the method. Presse Med. 1951;59(45):953–5.
Guillaume J, Janny P. Continuous intracranial manometry; importance of the method and first results. Rev Neurol (Paris). 1951;84(2):131–42.
Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36(149):1–193.
Miller JD, Butterworth JF, Gudeman SK, et al. Further experience in the management of severe head injury. J Neurosurg. 1981;54(3):289–99.
Valadka AB. Are external ventricular drains better than parenchymal intracranial pressure monitors in trauma patients? World Neurosurg. 2015;84(2):211–3.
Servadei F, Picetti E. Intracranial pressure monitoring and outcome in traumatic brain injury: the probe does matter? World Neurosurg. 2015;83(5):732–3.
Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121–30.
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82 discussion -82.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–7.
Lai HY, Lee CH, Lee CY. The intracranial volume pressure response in increased intracranial pressure patients: clinical significance of the volume pressure indicator. PLoS ONE. 2016;11(10):e0164263.
Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22(5):E1.
Marmarou A, Signoretti S, Fatouros PP, et al. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. 2006;104(5):720–30.
Hrabetova S, Nicholson C. Contribution of dead-space microdomains to tortuosity of brain extracellular space. Neurochem Int. 2004;45(4):467–77.
Matsuoka Y, Hossmann KA. Brain tissue osmolality after middle cerebral artery occlusion in cats. Exp Neurol. 1982;77(3):599–611.
Hossmann KA. Cortical steady potential, impedance and excitability changes during and after total ischemia of cat brain. Exp Neurol. 1971;32(2):163–75.
Hansen AJ, Olsen CE. Brain extracellular space during spreading depression and ischemia. Acta Physiol Scand. 1980;108(4):355–65.
Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–93.
Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45(4):545–52.
Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10.
Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol. 2015;273:57–68.
Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–340.
Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37(11):620–8.
Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978;48(3):332–44.
Czosnyka M, Czosnyka Z, Momjian S, Pickard JD. Cerebrospinal fluid dynamics. Physiol Meas. 2004;25(5):R51–76.
Balestreri M, Czosnyka M, Steiner LA, et al. Intracranial hypertension: what additional information can be derived from ICP waveform after head injury? Acta Neurochir (Wien). 2004;146(2):131–41.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
CC contributes to acquisition of data, analysis and interpretation of data, revising article; OD contributes to conception and design, acquisition of data, interpretation and analysis of data, derived equation for PE ratio, revising article and contributes equally to this manuscript; JCH contributes to interpretation of data, critically revising article; FR contributes to acquisition of data, analysis of data, revising article; GM contributes to interpretation of data, critically revising article; RP contributes to acquisition of data, critically revising article; GR contributes to conception and design, analysis and interpretation of data, drafting and revising article. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the Hadassah-Hebrew University Medical Center Institutional Review Board (IRB approval number 19384).
Rights and permissions
About this article
Cite this article
Candanedo, C., Doron, O., Hemphill, J.C. et al. Characterizing the Response to Cerebrospinal Fluid Drainage in Patients with an External Ventricular Drain: The Pressure Equalization Ratio. Neurocrit Care 30, 340–347 (2019). https://doi.org/10.1007/s12028-018-0612-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0612-y