Abstract
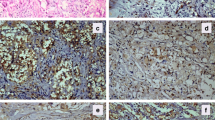
The etiology of polyarteritis nodosa (PAN) and localized PAN, including cutaneous arteritis (CA), remains unknown; however, initial endothelial damage has been implicated. The intima of the vasculitis lesions is predominantly infiltrated by innate-like bystander-activated CD8 T cells, in addition to the macrophages. Macrophages are among the major inflammatory cells involved in innate immunity and are classified into M1 and M2 subtypes. M1-type macrophages kill pathogens and cause inflammation, while M2-type macrophages promote the repair of tissues. Macrophage subtypes infiltrating in PAN and localized PAN vasculitis lesions have not yet been investigated. Innate immune response to a triggering factor on the endothelial cell surface may initiate CA pathogenesis. Thus, many M1-type macrophages may infiltrate in the intima during early CA. We assessed this hypothesis by immunohistochemical observation of macrophage phenotypes and polarization. Twenty-seven skin biopsy specimens from patients with CA were retrieved. Based on histology, we classified CA into four phases. The phenotypes of infiltrating macrophages in CA were evaluated by immunohistochemistry using antibodies against Iba-1, a pan-macrophage marker, and CD163, an M2-type macrophage marker. Our results showed that the ratio of CD163-positive M2-type macrophages to Iba1-positive macrophages was lower in the intima in the early stage of CA than in the later stage. In the media to adventitia, there was no significant difference in the ratios between these stages. These findings indicate that innate immunity is involved in the intima in the early stage of CA, suggesting that a trigger for CA might exist in endothelial cells.






Similar content being viewed by others
References
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. https://doi.org/10.1002/art.37715.
Ishibashi M, Chen KR. A morphological study of evolution of cutaneous polyarteritis nodosa. Am J Dermatopathol. 2008;30:319–26. https://doi.org/10.1097/dad.0b013e3181766190.
Coll-Vinent B, Cebrian M, Cid MC, Font C, Esparza J, Juan M, et al. Dynamic pattern of endothelial cell adhesion molecule expression in muscle and perineural vessels from patients with classic polyarteritis nodosa. Arthritis Rheum. 1998;41:435–44. https://doi.org/10.1002/1529-0131(199803)41:3%3C435::aid-art9%3E3.0.co;2-9.
Iinuma C, Waki M, Kawakami A, Yamaguchi M, Tomaru U, Sasaki N, et al. Establishment of a vascular endothelial cell-reactive type II NKT cell clone from a rat model of autoimmune vasculitis. Int Immunol. 2015;27:105–14. https://doi.org/10.1093/intimm/dxu088.
Kobayashi M, Ogawa E, Okuyama R, Kanno H. In vasculitis of small muscular arteries, activation of vessel-infiltrating CD8 T cells seems to be antigen-independent. Virchows Arch. 2018;472:271–9 https://link.springer.com/article/10.1007/s00428-017-2264-2.
Cid MC, Grau JM, Casademont J, Campo E, Coll-Vinent B, López-Soto A, et al. Immunohistochemical characterization of inflammatory cells and immunologic activation markers in muscle and nerve biopsy specimens from patients with systemic polyarteritis nodosa. Arthritis Rheum. 1994;37:1055–61. https://doi.org/10.1002/art.1780370711.
Francke ML, Mihaescu A, Chaubert P. Isolated necrotizing arteritis of the female genital tract: a clinicopathologic and immunohistochemical study of 11 cases. Int J Gynecol Pathol. 1998;17:193–200. https://doi.org/10.1097/00004347-199807000-00001.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. https://doi.org/10.1002/jcp.26429.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73 https://www.jimmunol.org/content/164/12/6166.
Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. https://doi.org/10.1189/jlb.0602325.
Huang X, Li Y, Fu M, Xin HB. Polarizing macrophages in vitro. In: Rousselet G, editor. Macrophages. Methods in Molecular Biology: Humana Press, New York; 2018, 1784.
Heusinkveld M, Van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216 https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-9-216.
Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. https://doi.org/10.1371/journal.pone.0080908.
O'Malley JT, Nadol JB Jr, McKenna MJ. Anti CD163+, Iba1+, and CD68+ cells in the adult human inner ear: normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol. 2016;37:99–108 https://insights.ovid.com/article/00129492-201601000-00016.
Nakagawa T, Ohnishi K, Kosaki Y, Saito Y, Horlad H, Fujiwara Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematopathol. 2017;57:31–6 https://www.jstage.jst.go.jp/article/jslrt/57/1/57_17017/_article.
Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24 https://onlinelibrary.wiley.com/doi/full/10.1002/path.2370.
Kawakami T, Takeuchi S, Soma Y. Serum levels of interleukin-6 in patients with cutaneous polyarteritis nodosa. Acta Derm Venereol. 2012;92:322–3 https://www.ingentaconnect.com/content/mjl/adv/2012/00000092/00000003/art00026?crawler=true&mimetype=application/pdf.
Ogata A, Kato Y, Higa S, Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29:258–67. https://doi.org/10.1080/14397595.2018.1546357.
Murakami M, Nishimoto N. The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Curr Opin Rheumatol. 2011;23:273–7. https://doi.org/10.1097/BOR.0b013e3283456797.
Schirmer M, Muratore F, Salvarani C. Tocilizumab for the treatment of giant cell arteritis. Expert Rev Clin Immunol. 2018;14:339–49. https://doi.org/10.1080/1744666X.2018.1468251.
Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L, Wang WQ, Zhang QB, Wu WZ, Wang L, Fan J, Tang ZY, Sun HC (2013) The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS One 2013; 8:e59771. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059771
Klein JL, Nguyen TT, Bien-Willner GA, Chen L, Foyil KV, Bartlett NL, et al. CD163 immunohistochemistry is superior to CD68 in predicting outcome in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141:381–7 https://academic.oup.com/ajcp/article/141/3/381/1766494.
Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, et al. Prognostic significance of CD68, CD163 and Folate receptor-beta positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15:4465–76 https://www.spandidos-publications.com/10.3892/etm.2018.5959.
Li J, Yu YF, Liu CH, Wang CM. Significance of M2 macrophages in glomerulonephritis with crescents. Pathol Res Pract. 2017;213:1215–20 https://www.sciencedirect.com/science/article/pii/S034403381630560X?via%3Dihub.
Li J, Liu CH, Gao B, Xu DL. Clinical-pathologic significance of CD163 positive macrophage in IgA nephropathy patients with crescents. Int J Clin Exp Med. 2015;8:9299–305 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4538096/.
Zhao L, David MZ, Hyjek E, Chang A, Meehan SM. M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol. 2015;10:54–62 https://cjasn.asnjournals.org/content/10/1/54.
de Souza AWS, van Timmeren M, Sanders JS, Stegeman C, Heeringa P, Kallenberg CGM, et al. M2 macrophage is the predominant phenotype in airways inflammatory lesions in patients with granulomatosis with polyangiitis. Arthritis Res Ther. 2017;19:100 https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-017-1310-4.
Acknowledgments
The authors thank Ms. Tomoko Nishizawa and Ms. Shizu Fujii for their technical assistance.
Funding
This research was partially supported by a grant from JSPS KAKENHI [Grant Number JP 18 K15077 to MK].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Mikiko Kobayashi, Yuki Matsumoto, and Hidetoshi Satomi. Data collection and analysis were performed by Mikiko Kobayashi, Maki Ohya, and Ayako Tateishi. Ichiro Ito and Hiroyuki Kanno assisted with data analysis and interpretation. The first draft of the manuscript was written by Mikiko Kobayashi, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was approved by the ethics committee of Shinshu University School of Medicine, Japan (No. 3136).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, M., Matsumoto, Y., Satomi, H. et al. The ratio of CD163-positive macrophages to Iba1-positive macrophages is low in the intima in the early stage of cutaneous arteritis. Immunol Res 68, 152–160 (2020). https://doi.org/10.1007/s12026-020-09140-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-020-09140-w