Abstract
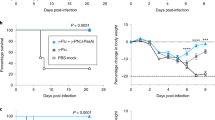
Limited protective effects of commercially available vaccines necessitate the development of novel pneumococcal vaccines. We recently reported a pneumococcal systemic vaccine containing two proteins, Pneumococcal surface protein A (PspA of family 1 and 2) and a bacterium-like particle-based pneumococcal mucosal vaccine containing PspA2 and PspA4 fragments, both eliciting broad protective immune responses. We had previously reported that subcutaneous (s.c.+s.c.+s.c.) immunization with the systemic vaccine induced more pronounced humoral serum IgG responses, while intranasal (i.n.+i.n.+i.n.) immunization with the mucosal vaccine elicited a more pronounced mucosal secretory IgA (sIgA) response. We hypothesized that a combinatorial administration of the two vaccines might elicit more pronounced and broader protective immune responses. Therefore, this study aimed to determine the efficacy of combinatorial prime-boost immunization using both systemic and mucosal vaccines for a pneumococcal infection. Combinatorial prime-boost immunization (s.c.+i.n. and i.n.+s.c.) induced not only IgG, but also mucosal sIgA production at high levels. Systemic priming and mucosal boosting immunization (s.c.+i.n.) provided markedly better protection than homologous prime-boost immunization (s.c.+s.c.+s.c. and i.n.+i.n.+i.n.). Moreover, it induced more robust Th1 and Th17 cell-mediated immune responses than mucosal priming and systemic boosting immunization (i.n.+s.c.). These results indicate that combinatorial prime-boost immunization potentially induces a robust systemic and mucosal immune response, making it an optimal alternative for maximum protection against lethal pneumococcal infections.





Similar content being viewed by others
References
Mitchell TJ. Streptococcus pneumoniae: infection, inflammation and disease. Adv Exp Med Biol. 2006;582:111–24.
Zhanel GG, Wolter KD, Karlowsky JA. Clinical cure rates in subjects treated with azithromycin for community-acquired respiratory tract infections caused by azithromycin-susceptible or azithromycin-resistant Streptococcus pneumoniae: analysis of phase 3 clinical trial data-authors' response. J Antimicrob Chemother. 2015;70:3170–1.
Bryce J, Boschi-Pinto C, Shibuya K, Black RE, Group WHOCHER. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52.
O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902.
Mera R, Miller LA, Fritsche TR, Jones RN. Serotype replacement and multiple resistance in Streptococcus pneumoniae after the introduction of the conjugate pneumococcal vaccine. Microb Drug Resist. 2008;14:101–7.
Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. 2010;28:5513–23.
Deng X, Church D, Vanderkooi OG, Low DE, Pillai DR. Streptococcus pneumoniae infection: a Canadian perspective. Expert Rev Anti-Infect Ther. 2013;11:781–91.
Tai SS. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol. 2006;32:139–53.
Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011;23:407–13.
Ochs MM, Williams K, Sheung A, Lheritier P, Visan L, Rouleau N, et al. A bivalent pneumococcal histidine triad protein D-choline-binding protein A vaccine elicits functional antibodies that passively protect mice from Streptococcus pneumoniae challenge. Hum Vaccin Immunother. 2016;12:2946–52.
Xu Q, Pryharski K, Pichichero ME. Trivalent pneumococcal protein vaccine protects against experimental acute otitis media caused by Streptococcus pneumoniae in an infant murine model. Vaccine. 2017;35:337–44.
Odutola A, Ota MOC, Antonio M, Ogundare EO, Saidu Y, Foster-Nyarko E, et al. Efficacy of a novel, protein-based pneumococcal vaccine against nasopharyngeal carriage of Streptococcus pneumoniae in infants: a phase 2, randomized, controlled, observer-blind study. Vaccine. 2017;35:2531–42.
Orihuela CJ, Radin JN, Sublett JE, Gao G, Kaushal D, Tuomanen EI. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun. 2004;72:5582–96.
Ren B, Szalai AJ, Hollingshead SK, Briles DE. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun. 2004;72:114–22.
Hollingshead SK, Becker R, Briles DE. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000;68:5889–900.
Daniels CC, Coan P, King J, Hale J, Benton KA, Briles DE, et al. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun. 2010;78:2163–72.
Vadesilho CF, Ferreira DM, Gordon SB, Briles DE, Moreno AT, Oliveira ML, et al. Mapping of epitopes recognized by antibodies induced by immunization of mice with PspA and PspC. Clin Vaccine Immunol. 2014;21:940–8.
Hollingshead SK, Baril L, Ferro S, King J, Coan P, Briles DE, et al. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol. 2006;55:215–21.
Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ. PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19:891–6.
Moreno AT, Oliveira ML, Ferreira DM, Ho PL, Darrieux M, Leite LC, et al. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin Vaccine Immunol. 2010;17:439–46.
Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182:1694–701.
Qian J, Yao K, Xue L, Xie G, Zheng Y, Wang C, et al. Diversity of pneumococcal surface protein A (PspA) and relation to sequence typing in Streptococcus pneumoniae causing invasive disease in Chinese children. Eur J Clin Microbiol Infect Dis. 2012;31:217–23.
Brandileone MC, Andrade AL, Teles EM, Zanella RC, Yara TI, Di Fabio JL, et al. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine. 2004;22:3890–6.
Yu J, Chen X, Li B, Gu T, Meng X, Kong W, et al. A pneumococcal vaccine combination with two proteins containing PspA families 1 and 2 can potentially protect against a wide range of Streptococcus pneumoniae strains. Immunol Res. 2018;66:528–36.
Ramirez K, Ditamo Y, Rodriguez L, Picking WL, van Roosmalen ML, Leenhouts K, et al. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunol. 2010;3:159–71.
Van Braeckel-Budimir N, Haijema BJ, Leenhouts K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol. 2013;4:282.
Keijzer C, Haijema BJ, Meijerhof T, Voorn P, de Haan A, Leenhouts K, et al. Inactivated influenza vaccine adjuvanted with bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine. 2014;32:2904–10.
Joris B, Englebert S, Chu CP, Kariyama R, Daneo-Moore L, Shockman GD, et al. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol Lett. 1992;70:257–64.
Bateman A, Bycroft M. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J Mol Biol. 2000;299:1113–9.
Steen A, Palumbo E, Deghorain M, Cocconcelli PS, Delcour J, Kuipers OP, et al. Autolysis of Lactococcus lactis is increased upon D-alanine depletion of peptidoglycan and lipoteichoic acids. J Bacteriol. 2005;187:114–24.
Lu J, Guo J, Wang D, Yu J, Gu T, Jiang C, et al. Broad protective immune responses elicited by bacterium-like particle-based intranasal pneumococcal particle vaccine displaying PspA2 and PspA4 fragments. Hum Vaccin Immunother. 2019;15:371–80.
Yu J, Li B, Chen X, Lu J, Wang D, Gu T, et al. Comparison of immunogenicity and protection of two pneumococcal protein vaccines based on PsaA and PspA. Infect Immun. 2018;86.
Lu J, Sun T, Wang D, Dong Y, Xu M, Hou H, et al. Protective immune responses elicited by fusion protein containing PsaA and PspA fragments. Immunol Investig. 2015;44:482–96.
Audouy SA, van Roosmalen ML, Neef J, Kanninga R, Post E, van Deemter M, et al. Lactococcus lactis GEM particles displaying pneumococcal antigens induce local and systemic immune responses following intranasal immunization. Vaccine. 2006;24:5434–41.
Mukerji R, Mirza S, Roche AM, Widener RW, Croney CM, Rhee DK, et al. Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine. J Immunol. 2012;189:5327–35.
Ren B, Li J, Genschmer K, Hollingshead SK, Briles DE. The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro. Clin Vaccine Immunol. 2012;19:1574–82.
Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Oishi K, et al. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol. 2010;185:1755–62.
Bruna-Romero O, Rocha CD, Tsuji M, Gazzinelli RT. Enhanced protective immunity against malaria by vaccination with a recombinant adenovirus encoding the circumsporozoite protein of Plasmodium lacking the GPI-anchoring motif. Vaccine. 2004;22:3575–84.
Nganou-Makamdop K, van Roosmalen ML, Audouy SA, van Gemert GJ, Leenhouts K, Hermsen CC, et al. Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar J. 2012;11:50.
Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–51.
Lu S. Two is better than one. Lancet Infect Dis. 2011;11:889–91.
Qiu Y, Zhang X, Wang H, Zhang X, Mo Y, Sun X, et al. Heterologous prime-boost immunization with live SPY1 and DnaJ protein of Streptococcus pneumoniae induces strong Th1 and Th17 cellular immune responses in mice. J Microbiol. 2017;55:823–9.
Feunou PF, Kammoun H, Debrie AS, Locht C. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine. 2014;32:4281–8.
Chen X, Li B, Yu J, Zhang Y, Mo Z, Gu T, et al. Comparison of four adjuvants revealed the strongest protection against lethal pneumococcal challenge following immunization with PsaA-PspA fusion protein and AS02 as adjuvant. Med Microbiol Immunol. 2019;208:215–26.
Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159.
Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–909.
Xu Q, Surendran N, Verhoeven D, Klapa J, Ochs M, Pichichero ME. Trivalent pneumococcal protein recombinant vaccine protects against lethal Streptococcus pneumoniae pneumonia and correlates with phagocytosis by neutrophils during early pathogenesis. Vaccine. 2015;33:993–1000.
Elhaik Goldman S, Dotan S, Talias A, Lilo A, Azriel S, Malka I, et al. Streptococcus pneumoniae fructose-1,6-bisphosphate aldolase, a protein vaccine candidate, elicits Th1/Th2/Th17-type cytokine responses in mice. Int J Mol Med. 2016;37:1127–38.
Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One. 2011;6:e25558.
Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8:627–39.
Acknowledgments
We gratefully acknowledge Editage (www.editage.cn) for the editorial support in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All mouse experiments in this paper were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Guo, X., Guo, M. et al. Combined prime-boost immunization with systemic and mucosal pneumococcal vaccines based on Pneumococcal surface protein A to enhance protection against lethal pneumococcal infections. Immunol Res 67, 398–407 (2019). https://doi.org/10.1007/s12026-019-09107-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-019-09107-6