Abstract
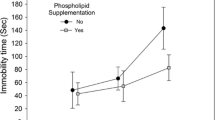
Vaccine adjuvants and vaccines may induce autoimmune and inflammatory manifestations in susceptible individuals. To date most human vaccine trials utilize aluminum (Al) adjuvants as placebos despite much evidence showing that Al in vaccine-relevant exposures can be toxic to humans and animals. We sought to evaluate the effects of Al adjuvant and the HPV vaccine Gardasil versus the true placebo on behavioral and inflammatory parameters in female mice. Six-week-old C57BL/6 female mice were injected with either, Gardasil, Gardasil + pertussis toxin (Pt), Al hydroxide, or, vehicle control in amounts equivalent to human exposure. At 7.5 months of age, Gardasil and Al-injected mice spent significantly more time floating in the forced swimming test (FST) in comparison with vehicle-injected mice (Al, p = 0.009; Gardasil, p = 0.025; Gardasil + Pt, p = 0.005). The increase in floating time was already highly significant at 4.5 months of age for the Gardasil and Gardasil + Pt group (p ≤ 0.0001). No significant differences were observed in the number of stairs climbed in the staircase test which measures locomotor activity. These results indicate that differences observed in the FST were unlikely due to locomotor dysfunction, but rather due to depression. Moreover, anti-HPV antibodies from the sera of Gardasil and Gardasil + Pt-injected mice showed cross-reactivity with the mouse brain protein extract. Immunohistochemistry analysis revealed microglial activation in the CA1 area of the hippocampus of Gardasil-injected mice. It appears that Gardasil via its Al adjuvant and HPV antigens has the ability to trigger neuroinflammation and autoimmune reactions, further leading to behavioral changes.




Similar content being viewed by others
Abbreviations
- Al:
-
Aluminum
- ASIA:
-
Autoimmune/autoinflammatory syndrome induced by adjuvants
- β2-GPI:
-
β2-Glycoprotein I
- FST:
-
Forced swimming test
- HPV:
-
Human papilloma virus
- Pt:
-
Pertussis toxin
- U. S FDA:
-
United States Food and Drug Administration
References
Marra F, et al. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. 2013;7(4):584–603. doi:10.1111/irv.12000.
Perricone C, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. doi:10.1016/j.jaut.2013.10.004.
Tomljenovic L, Shaw CA. No autoimmune safety signal after vaccination with quadrivalent HPV vaccine Gardasil? J Intern Med. 2012;272(5):514–5. doi:10.1111/j.1365-2796.2012.02551.x.
Blitshteyn S. Postural tachycardia syndrome following human papillomavirus vaccination. Eur J Neurol. 2014;21(1):135–9. doi:10.1111/ene.12272.
Poser CM, Behan PO. Late onset of Guillain-Barre syndrome. J Neuroimmunol. 1982;3(1):27–41.
Ryan AM, et al. Atypical presentation of macrophagic myofasciitis 10 years post vaccination. Neuromuscul Disord. 2006;16(12):867–9. doi:10.1016/j.nmd.2006.07.017.
Exley C. Aluminium-based adjuvants should not be used as placebos in clinical trials. Vaccine. 2011;29(50):9289. doi:10.1016/j.vaccine.2011.08.062.
Lujan L, et al. Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in commercial sheep. Immunol Res. 2013;56(2–3):317–24. doi:10.1007/s12026-013-8404-0.
Couette M, et al. Long-term persistence of vaccine-derived aluminum hydroxide is associated with chronic cognitive dysfunction. J Inorg Biochem. 2009;103(11):1571–8. doi:10.1016/j.jinorgbio.2009.08.005.
Gherardi RK, et al. Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front Neurol. 2015;6:4. doi:10.3389/fneur.2015.00004.
Rigolet M, et al. Clinical features in patients with long-lasting macrophagic myofasciitis. Front Neurol. 2014;5:230. doi:10.3389/fneur.2014.00230.
Marrack P, et al. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–93. doi:10.1038/nri2510.
Shaw CA, Petrik MS. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem. 2009;103(11):1555–62. doi:10.1016/j.jinorgbio.2009.05.019.
Li X, et al. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomedicine. 2009;5(4):473–9.
Zhu Y, et al. Immunotoxicity of aluminum. Chemosphere. 2014;104:1–6. doi:10.1016/j.chemosphere.2013.10.052.
Chen L, et al. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol. 2008;3(4):286–95. doi:10.1007/s11481-008-9131-5.
Eldred BE, et al. Vaccine components and constituents: responding to consumer concerns. Med J Aust. 2006;184(4):170–5.
Shoenfeld Y, et al. Vaccination as an additional player in the mosaic of autoimmunity. Clin Exp Rheumatol. 2000;18(2):181–4.
Shoenfeld Y. Infections, vaccines and autoimmunity. Lupus. 2009;18(13):1127–8. doi:10.1177/0961203309351081.
Tomljenovic L, Shaw CA. Human papillomavirus (HPV) vaccine policy and evidence-based medicine: Are they at odds? Ann Med. 2013;45(2):182–93. doi:10.3109/07853890.2011.645353.
Garland SM, et al. (FUTURE) I Investigators) Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi:10.1056/NEJMoa061760.
Munoz N, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–57. doi:10.1016/S0140-6736(09)60691-7.
Can A, et al. The mouse forced swim test. J Vis Exp. 2012;59:e3638. doi:10.3791/3638.
Tordera RM, et al. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1). Eur J Neurosci. 2007;25:281–90.
Katzav A, et al. Hyperactivity in a mouse model of the antiphospholipid syndrome. Lupus. 2001;10:496–9.
Brown JA, et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Human Mol Genet. 2010;19(22):4515–28.
Harper DM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–55. doi:10.1016/S0140-6736(06)68439-0.
Villa LL, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–8. doi:10.1016/S1470-2045(05)70101-7.
Mao C, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107(1):18–27. doi:10.1097/01.AOG.0000192397.41191.fb.
Group TFIS. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi:10.1056/NEJMoa061741.
Verstraeten T, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26(51):6630–8. doi:10.1016/j.vaccine.2008.09.049.
Guimaraes LE, et al. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015;100:190–209. doi:10.1016/j.phrs.2015.08.003.
Shaw CA, et al. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy. 2014;6(10):1055–71. doi:10.2217/imt.14.81.
Exley C. Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. Allergy Asthma Clin Immunol. 2014;10(1):4. doi:10.1186/1710-1492-10-4.
Passeri E, et al. Long-term follow-up of cognitive dysfunction in patients with aluminum hydroxide-induced macrophagic myofasciitis (MMF). J Inorg Biochem. 2011;105(11):1457–63. doi:10.1016/j.jinorgbio.2011.08.006.
Zivkovic I, et al. Induction of decreased fecundity by tetanus toxoid hyper-immunization in C57BL/6 mice depends on the applied adjuvant. Innate Immun. 2012;18(2):333–42. doi:10.1177/1753425911407361.
Agmon-Levin N, et al. Immunization with hepatitis B vaccine accelerates SLE-like disease in a murine model. J Autoimmun. 2014;54:21–32. doi:10.1016/j.jaut.2014.06.006.
Exley C, Birchall JD. The cellular toxicity of aluminium. J Theor Biol. 1992;159(1):83–98.
Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013;56(2–3):304–16. doi:10.1007/s12026-013-8403-1.
Offit PA, Jew RK. Addressing parents’ concerns: Do vaccines contain harmful preservatives, adjuvants, additives, or residuals? Pediatrics. 2003;112(6 Pt 1):1394–7.
Khan Z, et al. Slow CCL2-dependent translocation of biopersistent particles from muscle to brain. BMC Med. 2013;11:99. doi:10.1186/1741-7015-11-99.
Gherardi RK, et al. Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived aluminium hydroxide in muscle. Brain. 2001;124(Pt 9):1821–31.
Lee SH. Detection of human papillomavirus L1 gene DNA fragments in postmortem blood and spleen after Gardasil® vaccination—a case report. Adv Biosci Biotech. 2012;3:1214–24.
Walton JR. A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett. 2007;412(1):29–33. doi:10.1016/j.neulet.2006.08.093.
Xiu C, et al. Aluminum chloride- and norepinephrine-induced immunotoxicity on splenic lymphocytes by activating beta-AR/cAMP/PKA/NF-kappaB signal pathway in rats. Biol Trace Elem Res. 2014;162(1–3):168–74. doi:10.1007/s12011-014-0149-7.
Caulfield MJ, et al. Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Human Vaccin. 2007;3(4):139–45.
FDA. Food and Drug Administration. Inside clinical trials: testing medical products in people. (2009).
Kanduc D. Potential cross-reactivity between HPV16 L1 protein and sudden death-associated antigens. J Exp Ther Oncol. 2011;9(2):159–65.
Yonee C, et al. Association of acute cerebellar ataxia and human papilloma virus vaccination: a case report. Neuropediatrics. 2013;44(5):265–7. doi:10.1055/s-0033-1333873.
Kanduc D. Quantifying the possible cross-reactivity risk of an HPV16 vaccine. J Exp Ther Oncol. 2009;8(1):65–76.
van Bogaert L. Are the currently existing anti-human papillomavirus vaccines appropriate for the developing world? Ann Med Health Sci Res. 2013;3(3):306–12. doi:10.4103/2141-9248.117924.
Mendoza Plasencia Z, et al. Acute disseminated encephalomyelitis with tumefactive lesions after vaccination against human papillomavirus. Neurologia. 2010;25(1):58–9.
Wildemann B, et al. Acute disseminated encephalomyelitis following vaccination against human papilloma virus. Neurology. 2009;72(24):2132–3. doi:10.1212/WNL.0b013e3181aa53bb.
Sutton I, et al. CNS demyelination and quadrivalent HPV vaccination. Mult Scler. 2009;15(1):116–9.
Chang J, et al. Demyelinating disease and polyvalent human papilloma virus vaccination. J Neurol Neurosurg Psychiatry. 2011;82(11):1296–8. doi:10.1136/jnnp.2010.214924.
McCarthy JE, Filiano J. Opsoclonus Myoclonus after human papilloma virus vaccine in a pediatric patient. Parkinsonism Relat Disord. 2009;15(10):792–4. doi:10.1016/j.parkreldis.2009.04.002.
Menge T, et al. Neuromyelitis optica following human papillomavirus vaccination. Neurology. 2012;79(3):285–7. doi:10.1212/WNL.0b013e31825fdead.
DiMario FJ Jr, et al. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papilloma virus. J Child Neurol. 2010;25(3):321–7. doi:10.1177/0883073809349322.
Alvarez-Soria MJ, et al. Trastornos neurológicos desmielinizantes y vacunación del papilomavirus humano. Rev Neurol. 2011;52(8):472–6.
Zhu YZ, et al. impact of aluminum exposure on the immune system: a mini review. Environ Toxicol Pharmacol. 2013;35(1):82–7. doi:10.1016/j.etap.2012.11.009.
Debeer P, et al. Brachial plexus neuritis following HPV vaccination. Vaccine. 2008;26(35):4417–9. doi:10.1016/j.vaccine.2008.06.074.
Brinth LS, et al. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine. 2015;33(22):2602–5. doi:10.1016/j.vaccine.2015.03.098.
Kinoshita T, et al. Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine. Intern Med. 2014;53(19):2185–200.
Blitshteyn S. Postural tachycardia syndrome following human papillomavirus vaccination. Eur J Neurol. 2014;21:135–9. doi:10.1111/ene.12272.
Richards S, et al. Complex regional pain syndrome following immunisation. Arch Dis Child. 2012;97(10):913–5. doi:10.1136/archdischild-2011-301307.
Tomljenovic L, et al. Postural orthostatic tachycardia with chronic fatigue after HPV vaccination as part of the “autoimmune/autoinflammatory syndrome induced by adjuvants”: case report and literature review. J Investig Med High Impact Case Rep. 2014;. doi:10.1177/2324709614527812.
Martinez-Lavin M. Fibromyalgia-like illness in 2 girls after human papillomavirus vaccination. J Clin Rheumatol. 2014;20(7):392–3. doi:10.1097/RHU.0000000000000165.
Cerami C, et al. Autoimmune neuromyotonia following human papilloma virus vaccination. Muscle Nerve. 2013;47(3):466–7. doi:10.1002/mus.23648.
Colafrancesco S, et al. HPV vaccines and primary ovarian failure: another facet of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Am J Reprod Immunol. 2013;70(4):309–16.
Little DT, Grenville Ward HR. Adolescent premature ovarian insufficiency following human papillomavirus vaccination: a case series seen in general practice. J Investig Med High Impact Case Rep. 2014;. doi:10.1177/2324709614556129.
Melo Gomes S, et al. Vasculitis following HPV immunization. Rheumatology (Oxford). 2013;52(3):581–2. doi:10.1093/rheumatology/kes168.
Pugnet G, et al. Immune thrombocytopenic purpura following human papillomavirus vaccination. Vaccine. 2009;27(28):3690. doi:10.1016/j.vaccine.2009.04.004.
Della Corte C, et al. Autoimmune hepatitis type 2 following anti-papillomavirus vaccination in a 11-year-old girl. Vaccine. 2011;29(29):4654–6. doi:10.1016/j.vaccine.2011.05.002.
Das A, et al. Pancreatitis following human papillomavirus vaccination. Med J Aust. 2008;189(3):178.
Soldevilla HF, et al. Systemic lupus erythematosus following HPV immunization or infection? Lupus. 2012;21(2):158–61. doi:10.1177/0961203311429556.
Gatto M, et al. Human papillomavirus vaccine and systemic lupus erythematosus. Clin Rheumatol. 2013;32(9):1301–7. doi:10.1007/s10067-013-2266-7.
Anaya JM, et al. Autoimmune/auto-inflammatory syndrome induced by adjuvants (ASIA) after quadrivalent human papillomavirus vaccination in Colombians: a call for personalised medicine. Clin Exp Rheumatol. 2015;33(4):545–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yehuda Shoenfeld has acted as a consultant for the no-fault US National Vaccine Injury Compensation Program. L.T. has served as an expert witness in cases involving adverse reactions following qHPV vaccine administration. The other co-authors declare no competing interests.
Funding
This work was supported by the grants from the Dwoskin Foundation Ltd.
Rights and permissions
About this article
Cite this article
Inbar, R., Weiss, R., Tomljenovic, L. et al. Behavioral abnormalities in female mice following administration of aluminum adjuvants and the human papillomavirus (HPV) vaccine Gardasil. Immunol Res 65, 136–149 (2017). https://doi.org/10.1007/s12026-016-8826-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-016-8826-6