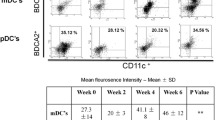
Abstract
Pattern recognition receptors (PRRs) are involved in direct recognition of viruses, promoting cellular activation and the production of pro-inflammatory cytokines. However, despite the reduced systemic immune activation described in HIV-1-exposed seronegatives (HESNs), few studies have focused on determining the relationship between PRR expression and cytokine production. We have aimed here to evaluate the expression level of PRRs and cytokines in HESNs, HIV-1 patients and healthy donors. Basal PRR expression levels in PBMCs, dendritic cells (DCs) and monocytes, and plasma cytokine levels as well as the PRR ligand-induced cytokine productions were determined by flow cytometry, qPCR and ELISA. Higher TLR2/4 expression in DCs and monocytes from HESNs was observed. Nevertheless, TLR4/8, NOD2 and RIG-I mRNA levels were lower in PBMCs from HESNs than HIV-1-infected patients. Comparable IL-1β, IL-18 and TNF-α mRNA levels were observed among the groups examined; however, at the protein level, production of IL-1β, IL-6 and IL-10 was significantly lower in plasma from HESNs than from HIV-1-infected patients. Our results suggest that exposure to HIV-1 without infection could be associated with reduced basal pro-inflammatory responses. Further studies are required to define the cell subsets responsible for these differences and the role of PRRs on protection against HIV-1 infection.






Similar content being viewed by others
References
HIV, AIDS JUNPo, (UNAIDS). 2013 UNAIDS Report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013.
Fowke KR, Nagelkerke NJ, Kimani J, Simonsen JN, Anzala AO, Bwayo JJ, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–51.
Makedonas G, Bruneau J, Alary M, Tsoukas CM, Lowndes CM, Lamothe F, et al. Comparison of HIV-specific CD8 T-cell responses among uninfected individuals exposed to HIV parenterally and mucosally. AIDS. 2005;19:251–9.
Begaud E, Chartier L, Marechal V, Ipero J, Leal J, Versmisse P, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35.
Horton RE, McLaren PJ, Fowke K, Kimani J, Ball TB. Cohorts for the study of HIV-1-exposed but uninfected individuals: benefits and limitations. J Infect Dis. 2010;202(Suppl 3):S377–81.
Palsson-McDermott EM, O’Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–44.
Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60.
Hernandez JC, Arteaga J, Paul S, Kumar A, Latz E, Urcuqui-Inchima S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Res Hum Retrovir. 2011;27:1099–109.
Hernandez JC, Stevenson M, Latz E, Urcuqui-Inchima S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS Res Hum Retrovir. 2012;28:1313–28.
Biasin M, Piacentini L, Lo Caputo S, Naddeo V, Pierotti P, Borelli M, et al. TLR activation pathways in HIV-1-exposed seronegative individuals. J Immunol. 2010;184:2710–7.
Barton GM. Viral recognition by toll-like receptors. Semin Immunol. 2007;19:33–40.
Sironi M, Biasin M, Cagliani R, Forni D, De Luca M, Saulle I, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J Immunol. 2012;188:818–23.
Yao XD, Omange RW, Henrick BM, Lester RT, Kimani J, Ball TB, et al. Acting locally: innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol. 2013.
Hernandez JC, Giraldo DM, Paul S, Urcuqui-Inchima S. Involvement of neutrophil hyporesponse and the role of toll-like receptors in human immunodeficiency virus 1 protection. PLoS ONE. 2015;10:e0119844.
Berkley S, Bertram K, Delfraissy JF, Draghia-Akli R, Fauci A, Hallenbeck C, et al. The 2010 scientific strategic plan of the Global HIV Vaccine Enterprise. Nat Med. 2010;16:981–9.
McLaren PJ, Ball TB, Wachihi C, Jaoko W, Kelvin DJ, Danesh A, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;202(Suppl 3):S339–44.
Koning FA, Otto SA, Hazenberg MD, Dekker L, Prins M, Miedema F, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005;175:6117–22.
Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–22.
Lajoie J, Juno J, Burgener A, Rahman S, Mogk K, Wachihi C, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012;5:277–87.
Card CM, Ball TB, Fowke KR. Immune quiescence: a model of protection against HIV infection. Retrovirology. 2013;10:141.
Jennes W, Evertse D, Borget MY, Vuylsteke B, Maurice C, Nkengasong JN, et al. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clin Exp Immunol. 2006;143:435–44.
Chege D, Chai Y, Huibner S, Kain T, Wachihi C, Kimani M, et al. Blunted IL17/IL22 and pro-inflammatory cytokine responses in the genital tract and blood of HIV-exposed, seronegative female sex workers in Kenya. PLoS ONE. 2012;7:e43670.
Huik K, Avi R, Pauskar M, Kallas E, Jogeda EL, Karki T, et al. Association between TLR3 rs3775291 and resistance to HIV among highly exposed Caucasian intravenous drug users. Infect Genet Evol. 2013;20:78–82.
Goh WC, Markee J, Akridge RE, Meldorf M, Musey L, Karchmer T, et al. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–57.
Zapata W, Rodriguez B, Weber J, Estrada H, Quinones-Mateu ME, Zimermman PA, et al. Increased levels of human β-defensins mRNA in sexually HIV-1 exposed but uninfected individuals. Curr HIV Res. 2008;6:531–8.
Torres S, Hernandez JC, Giraldo D, Arboleda M, Rojas M, Smit JM, et al. Differential expression of toll-like receptors in dendritic cells of patients with dengue during early and late acute phases of the disease. PLoS Negl Trop Dis. 2013;7:e2060.
Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–91.
Jost S, Altfeld M. Evasion from NK cell-mediated immune responses by HIV-1. Microb Infect. 2012;14:904–15.
Freguja R, Gianesin K, Del Bianco P, Malacrida S, Rampon O, Zanchetta M, et al. Polymorphisms of innate immunity genes influence disease progression in HIV-1-infected children. AIDS. 2012;26:765–8.
Pine SO, McElrath MJ, Bochud PY. Polymorphisms in toll-like receptor 4 and toll-like receptor 9 influence viral load in a seroincident cohort of HIV-1-infected individuals. AIDS. 2009;23:2387–95.
Biasin M, Caputo SL, Speciale L, Colombo F, Racioppi L, Zagliani A, et al. Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect Dis. 2000;182:1365–74.
Jennes W, Sawadogo S, Koblavi-Deme S, Vuylsteke B, Maurice C, Roels TH, et al. Cellular human immunodeficiency virus (HIV)-protective factors: a comparison of HIV-exposed seronegative female sex workers and female blood donors in Abidjan, Cote d’Ivoire. J Infect Dis. 2003;187:206–14.
Tran HK, Chartier L, Troung LX, Nguyen NN, Fontanet A, Barre-Sinoussi FE, et al. Systemic immune activation in HIV-1-exposed uninfected Vietnamese intravascular drug users. AIDS Res Hum Retrovir. 2006;22:255–61.
Montoya CJ, Velilla PA, Chougnet C, Landay AL, Rugeles MT. Increased IFN-γ production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006;120:138–46.
Kaushik S, Teque F, Patel M, Fujimura SH, Schmidt B, Levy JA. Plasmacytoid dendritic cell number and responses to toll-like receptor 7 and 9 agonists vary in HIV Type 1-infected individuals in relation to clinical state. AIDS Res Hum Retrovir. 2013;29:501–10.
Singla A, Jacobs R, Schmidt R, Wanchu A, Arora S. Increased activity of NK cells and plasmacytoid dendritic cells in HIV-exposed seronegative (ESN) individuals. World J AIDS. 2012;2:6–16.
Taborda NA, Hernandez JC, Lajoie J, Juno JA, Kimani J, Rugeles MT, et al. Low expression of activation and inhibitory molecules on NK cells and CD4 T cells is associated with viral control. AIDS Res Hum Retrovir. 2015 (Epub ahead of print).
Tomescu C, Seaton KE, Smith P, Taylor M, Tomaras GD, Metzger DS, et al. Innate activation of MDC and NK cells in high-risk HIV-1-exposed seronegative IV-drug users who share needles when compared with low-risk nonsharing IV-drug user controls. J Acquir Immune Defic Syndr. 2015;68:264–73.
Songok EM, Luo M, Liang B, McLaren P, Kaefer N, Apidi W, et al. Microarray analysis of HIV resistant female sex workers reveal a gene expression signature pattern reminiscent of a lowered immune activation state. PLoS ONE. 2012;7:e30048.
Wu JQ, Sasse TR, Wolkenstein G, Conceicao V, Saksena MM, Soedjono M, et al. Transcriptome analysis of primary monocytes shows global down-regulation of genetic networks in HIV viremic patients versus long-term non-progressors. Virology. 2013;435:308–19.
Ghosh M, Shen Z, Fahey JV, Crist SG, Patel M, Smith JM, et al. Pathogen recognition in the human female reproductive tract: expression of intracellular cytosolic sensors NOD1, NOD2, RIG-1, and MDA5 and response to HIV-1 and Neisseria gonorrhea. Am J Reprod Immunol. 2013;69:41–51.
Cote SC, Plante A, Tardif MR, Tremblay MJ. Dectin-1/TLR2 and NOD2 agonists render dendritic cells susceptible to infection by X4-using HIV-1 and promote cis-infection of CD4(+) T cells. PLoS ONE. 2013;8:e67735.
Gay C, Dibben O, Anderson JA, Stacey A, Mayo AJ, Norris PJ, et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS ONE. 2011;6:e19617.
Haissman JM, Vestergaard LS, Sembuche S, Erikstrup C, Mmbando B, Mtullu S, et al. Plasma cytokine levels in Tanzanian HIV-1-infected adults and the effect of antiretroviral treatment. J Acquir Immune Defic Syndr. 2009;52:493–7.
Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, et al. Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology. 2013;10:119.
Taborda NA, Catano JC, Delgado JC, Rugeles MT, Montoya CJ. Higher SLPI expression, lower immune activation, and increased frequency of immune cells in a cohort of Colombian HIV-1 controllers. J Acquir Immune Defic Syndr. 2012;60:12–9.
Yonkers NL, Rodriguez B, Asaad R, Lederman MM, Anthony DD. Systemic immune activation in HIV infection is associated with decreased MDC responsiveness to TLR ligand and inability to activate naive CD4 T-cells. PLoS ONE. 2011;6:e23884.
Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25:1823–32.
Acknowledgments
The authors thank the patients and control individuals who participated in this study and the collaboration of the personnel from the institutions where the patients were recruited. We acknowledge the help of Anne-Lise Haenni for critically reading and reviewing of the manuscript. The authors also acknowledge the technical assistance of Diana Giraldo, Lanie Ruiz and Maria Patricia García.
Author contributions
J.C.H. and S.U.I. were responsible for the conception and design of the project, as well as the acquisition, analysis and interpretation of the data. The authors read and approved the final manuscript.
Sources of financial
This study was funded by COLCIENCIAS, Grant No. 111549326099, and Universidad de Antioquia (estrategia de sostenibilidad, 2014–2015). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supporting information 2
HESNs express lower NOD2 mRNA levels than HIV-1-infected patients. The mRNA of several NOD-like receptors and RIG-I-like receptors was measured using qRT-PCR and normalized with the housekeeping genes β-actin, GAPDH and β2-microglobulin. Relative units of transcripts versus average of housekeeping gene transcripts are shown as median and range. Comparisons were made by the Kruskal–Wallis test and Dunn’s post-tests. The level of significance was p < 0.05 (*). Median with ranges is shown. Healthy donors (n = 13), HIV-1-infected patients (n = 31) and HESNs (n = 11) (PDF 46 kb)
Rights and permissions
About this article
Cite this article
Hernandez, J.C., St Laurent, G. & Urcuqui-Inchima, S. HIV-1-exposed seronegative individuals show alteration in TLR expression and pro-inflammatory cytokine production ex vivo: An innate immune quiescence status?. Immunol Res 64, 280–290 (2016). https://doi.org/10.1007/s12026-015-8748-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8748-8