Abstract
Purpose
To evaluate the effect of oral alpha-lipoic acid (ALA) ± palmitoyl-ethanolamide (PEA) on neuropathic symptoms in patients with diabetic peripheral neuropathy (DPN) and to identify factors related to the efficacy of the treatment.
Methods
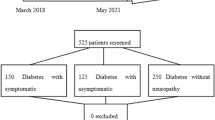
This is a retrospective observational pilot study evaluating 49 patients with diabetes and positive Neuropathy Symptoms Score (NSS). Clinical and biochemical variables, including NSS, were compared between untreated patients and patients treated with oral 600 mg/day ALA ± 600 mg/day PEA at baseline (first occurrence of NSS ≥ 3) and at least 2 months after baseline. Number of days between treatment initiation and symptoms’ relief and related factors were also investigated.
Results
Thirty subjects were treated with ALA ± PEA and 19 subjects did not receive any specific treatment for neuropathy symptoms. Follow-up visits occurred after 98 ± 46 days. NSS significantly decreased in patients treated with ALA ± PEA (5.4 ± 1.3 at baseline vs. 1.7 ± 2.4 at follow-up, p < 0.001), but not in untreated patients (p = 0.164). Subjects treated with ALA ± PEA reported a mean time from treatment initiation to symptoms’ relief of 18.4 ± 9.0 days. The number of days of treatment needed for symptoms’ relief was inversely related to HDL-cholesterol levels (r = −0.503, p = 0.010) and to eGFR (r = −0.428, p = 0.033), whereas there was no significant relationship between time to symptoms’ relief and age, HbA1c, lipid profile and the severity of symptoms at baseline.
Conclusions
This study documents that oral administration of ALA ± PEA helps in controlling neuropathy symptoms in diabetes. Moreover, our data show that higher HDL-c levels and better renal function are associated to a faster therapeutic effect, suggesting them as biomarkers of response to therapy with ALA ± PEA.


Similar content being viewed by others
References
E. Maddaloni, F. Sabatino, In vivo corneal confocal microscopy in diabetes: where we are and where we can get. World J. Diabetes 7(17), 406–411 (2016)
E. Maddaloni et al. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet. Med. 32, 262–266 (2015)
Z. Iqbal et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin. Ther. 40, 828–849 (2018)
D.A. Greene, A.A.F. Sima, M.J. Stevens, E.L. Feldman, S.A. Lattimer, Complications: neuropathy, pathogenetic considerations. Diabetes Care 15, 1902–1925 (1992)
S.D. Solomon et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 40, 412–418 (2017)
E. Maddaloni, R. Buzzetti, Why only macro and not micro in type 2 diabetes? Time to change the goals of clinical trials in diabetes. Diabetes/Metab. Res. Rev 34(6), e3012 (2018). https://doi.org/10.1002/dmrr.3012
M. Brownlee, The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005)
R. Pop-Busui, A. Sima, M. Stevens, Review: diabetic neuropathy and oxidative stress. Diabetes. Metab. Res. Rev. 22, 257–273 (2006)
N. Papanas, D. Ziegler, Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin. Pharmacother. 15, 2721–2731 (2014)
N. Vallianou, A. Evangelopoulos, P. Koutalas, Alpha-lipoic acid and diabetic neuropathy. Rev. Diabet. Stud. 6, 230–236 (2009)
N.A. Darmani et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology. 48, 1154–1163 (2005)
S. Petrosino, V.Di Marzo, The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 174, 1349–1365 (2017)
J.W.G. Meijer, A.J. Smit, E.V. Sonderen, J.W. Groothoff, W.H. Eisma, T.P. Links, Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the diabetic neuropathy symptom score. Diabet. Med. 19, 962–965 (2002)
C.A. Abbott et al. The North-West Diabetes Foot Care Study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet. Med. 19, 377–384 (2002)
D. Ziegler, H. Nowak, P. Kempler, P. Vargha, P.A. Low, Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a meta-analysis. Diabet. Med. 21, 114–121 (2004)
T. Han, J. Bai, W. Liu, Y. Hu, A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 167, 465–471 (2012)
D. Ziegler et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy. Diabetes Care 29, 2365–2370 (2006)
K.J. Ruhnau et al. Effects of 3-week oral treatment with the antioxidant thioctic acid (α- lipoic acid) in symptomatic diabetic polyneuropathy. Diabet. Med. 16, 1040–1043 (1999)
G.S. Mijnhout, B.J. Kollen, A. Alkhalaf, N. Kleefstra, H.J.G. Bilo, Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012, 456279 (2012)
P.A. Low, K.K. Nickander, H.J. Tritschler, The roles of oxidative stress and antioxidant. Treatment in experimental diabetic neuropathy. Diabetes 46(Suppl 2), S38–S42 (1997)
L. Rochette, S. Ghibu, A. Muresan, C. Vergely, Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 93, 1021–1027 (2015)
D. Ziegler, H. Schatz, F. Conrad, F.A. Gries, H. Ulrich, G. Reichel, Effects of treatment with the antioxidant α-lipoic acid on cardiac autonomic neuropathy in NIDDM patients: a 4-month randomized controlled multicenter trial (DEKAN study). Diabetes Care 20, 369–373 (1997)
F. Guida et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 8, 47 (2015)
L. Di Cesare Mannelli et al. Antineuropathic profile of N-Palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS ONE 10, e0128080 (2015)
Antonella Paladini, MariellaFusco, Teresa Cenacchi, P.Carlo Schievano, Alba Piroli, Giustino Varrassi, Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: a pooled data meta-analysis. Pain Physician 19, 11–24 (2016)
H.L. Hébert, A. Veluchamy, N. Torrance, B.H. Smith, Risk factors for neuropathic pain in diabetes mellitus. Pain 158, 560–568 (2017)
R. Pop-Busui, L. Roberts, S. Pennathur, M. Kretzler, F.C. Brosius, E.L. Feldman, The management of diabetic neuropathy in CKD. Am. J. Kidney Dis. 55, 365–385 (2010)
K.-A. Rye, P.J. Barter, Cardioprotective functions of HDL. J. Lipid Res. 55(2), 168–179 (2014).
E. Maddaloniet al. High density lipoprotein modulates osteocalcin expression in circulating monocytes: a potential protective mechanism for cardiovascular disease in type 1 diabetes. Cardiovasc. Diabetol. 16(1), 116 (2017)
S. Tesfaye et al. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 352, 341–350 (2005)
R.E. Maser et al. Epidemiological correlates of diabetic neuropathy. report from Pittsburgh epidemiology of diabetes complications study. Diabetes 38, 1456–1461 (1989)
M. Jaiswal, et al. Prevalence of and risk factors for diabetic peripheralneuropathy in youth with type 1 and type 2 diabetes: Search for diabetes in youth study. Diabetes Care 40, 1226–1232 (2017)
F. Kannenberg et al. Characterization of cholesterol homeostasis in telomeraseimmortalized tangier disease fibroblasts reveals marked phenotype variability. J. Biol. Chem. 288, 36936–36947 (2013)
J. Katz, N.B. Finnerup, R.H. Dworkin, Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology 70, 263–272 (2008)
Acknowledgements
This study was in part supported by an unrestricted grant from LJ Pharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of Campus Bio-Medico University of Rome, Italy. For this retrospective study, formal consent is not required. No patient identifiable information has been used in this study and only data from which identifying factors have been removed were used for statistical analysis.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pieralice, S., Vari, R., Minutolo, A. et al. Biomarkers of response to alpha-lipoic acid ± palmitoiletanolamide treatment in patients with diabetes and symptoms of peripheral neuropathy. Endocrine 66, 178–184 (2019). https://doi.org/10.1007/s12020-019-01917-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01917-w