Abstract
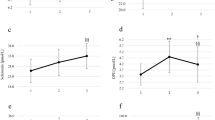
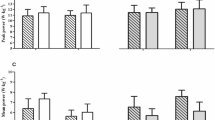
Bone and energy metabolisms regulation depends on a two-way street aimed at regulating energy utilization. Mountain ultra-marathons are highly demanding aerobic performances that deeply affect the whole body homeostasis. In this study we aimed to investigate and characterize the metabolic profile (in terms of hormones involved in energy metabolism), the inflammatory adipokines, and the bone turnover; in particular the osteocalcin-mediated response has been compared in experienced mountain ultra-marathons runners versus control subjects. Serum concentrations of specific markers of bone turnover (pro-collagen type I N-terminal propeptide, carboxylated/undercarboxylated osteocalcin), measured by enzyme-linked immunosorbent assay, and metabolic hormones (C-peptide, insulin, glucagon, glucagon-like peptide, gastric-inhibitory peptide, ghrelin, leptin, resistin, and visfatin), measured by fluorescent-based multiplex assay, were compared before and after a 65 km mountain ultra-marathons in 17 trained runners and 12 age-matched controls characterized by a low physical activity profile. After the mountain ultra-marathons, runners experienced a reduction in pro-collagen type I N-terminal propeptide, though it remained higher than in controls; while carboxylated osteocalcin remained unchanged. Among the metabolic hormones, only glucagon and leptin were different between runners and controls at rest. C-peptide and leptin decreased after the mountain ultra-marathons in runners; while glucagon, glucagon-like peptide 1, resistin, and visfatin were all increased. Uncarboxylated osteocalcin (and uncarboxylated/carboxylated osteocalcin ratio) was decreased and this highly correlated with insulin and C-peptide levels. In conditions of high energy expenditure, homeostasis is maintained at expenses of bone metabolism. Changes in the uncarboxylated osteocalcin clearly mark the global energy needs of the body.



Similar content being viewed by others
References
D.M. Bramble, D.E. Lieberman, Endurance running and the evolution of Homo. Nature 432(7015), 345–352 (2004)
World Health Organization. Global Database on Blood Safety: Report 2004–2005. World Health Organization, Geneva (2008)
Z.C. Thent, S. Das, L.J. Henry, Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One 8(11), e80436 (2013)
G. Lombardi, F. Sanchis-Gomar, S. Perego, V. Sansoni, G. Banfi, Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine (2015). [Epub ahead of print]. doi:10.1007/s12020-015-0834-0
H. Wallberg-Henriksson, J.R. Zierath, Metabolism. Exercise remodels subcutaneous fat tissue and improves metabolism. Nat. Rev. Endocrinol. 11(4), 198–200 (2015)
M. Gleeson, N.C. Bishop, D.J. Stensel, M.R. Lindley, S.S. Mastana, M.A. Nimmo, The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11(9), 607–615 (2011)
A. Sahin-Efe, F. Katsikeris, C.S. Mantzoros, Advances in adipokines. Metabolism 61(12), 1659–1665 (2012)
M.A. Nimmo, M. Leggate, J.L. Viana, J.A. King, The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 15(Suppl 3), 51–60 (2013)
G. Banfi, G. Lombardi, A. Colombini, G. Lippi, Bone metabolism markers in sports medicine. Sports Med. 40, 697–714 (2010)
J. Xu, G. Lombardi, W. Jiao, G. Banfi, Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sports Med. (2016). [Epub ahead of print]. doi:10.1007/s40279-016-0494-0
G. Lombardi, P. Lanteri, G. Graziani, A. Colombini, G. Banfi, R. Corsetti, Bone and energy metabolism parameters in professional cyclists during the Giro d’Italia 3-weeks stage race. PLoS One 7(7), e42077 (2012)
D. Grasso, R. Corsetti, P. Lanteri, C. Di Bernardo, A. Colombini, R. Graziani, G. Banfi, G. Lombardi, Bone-muscle unit activity, salivary steroid hormones profile, and physical effort over a 3-week stage race. Scand. J. Med. Sci. Sports 25(1), 70–80 (2015)
G. Lombardi, P. Lanteri, A. Colombini, M. Mariotti, G. Banfi, Sclerostin concentrations in athletes: role of load and gender. J. Biol. Regul. Homeost. Agents 26(1), 157–163 (2012)
K.J. Motyl, L.R. McCabe, A.V. Schwartz, Bone and glucose metabolism: a two-way street. Arch. Biochem. Biophys. 503(1), 2–10 (2010)
T. Otani, A. Mizokami, Y. Hayashi, J. Gao, Y. Mori, S. Nakamura, H. Takeuchi, M. Hirata, Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell. Signal. 27(3), 532–544 (2015)
G. Lombardi, S. Perego, L. Luzi, G. Banfi, A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine 48, 394–404 (2015)
G. Vernillo, A. Savoldelli, A. Zignoli, P. Trabucchi, B. Pellegrini, G.P. Millet, F. Schena, Influence of the world’s most challenging mountain ultra-marathon on energy cost and running mechanics. Eur. J. Appl. Physiol. 114(5), 929–939 (2014)
G. Vernillo, N. Rinaldo, A. Giorgi, F. Esposito, P. Trabucchi, G.P. Millet, F. Schena, Changes in lung function during an extreme mountain ultramarathon. Scand. J. Med. Sci. Sports 25(4), e374–e380 (2015)
G. Vernillo, A. Savoldelli, A. Zignoli, S. Skafidas, A. Fornasiero, A. La Torre, L. Bortolan, B. Pellegrini, F. Schena, Energy cost and kinematics of level, uphill and downhill running: fatigue-induced changes after a mountain ultramarathon. J. Sports Sci. 33(19), 1998–2005 (2015)
G.P. Millet, G.Y. Millet, Ultramarathon is an outstanding model for the study of adaptive responses to extreme load and stress. BMC Med. 10, 77 (2012)
I. Mujika, S. Padilla, Scientific bases for precompetition tapering strategies. Med. Sci. Sports Exerc. 35(7), 1182–1187 (2003)
ACSM: American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 8th edn., Philadephia, PA (2010)
G. Lombardi, P. Lanteri, A. Colombini, G. Banfi, Blood biochemical markers of bone turnover: pre-analytical and technical aspects of sample collection and handling. Clin. Chem. Lab. Med. 50(5), 771–789 (2012)
F. Curtin, P. Schulz, Multiple correlations and Bonferroni’s correction. Biol. Psychiatry 44(8), 775–777 (1998)
C. Fiuza-Luces, J.R. Ruiz, G. Rodriguez-Romo, C. Santiago, F. Gomez-Gallego, T. Yvert, A. Cano-Nieto, N. Garatachea, M. Moran, A. Lucia, Are ‘endurance’ alleles ‘survival’ alleles? Insights from the ACTN3 R577X polymorphism. PLoS One 6(3), e17558 (2011)
S.L. Booth, A. Centi, S.R. Smith, C. Gundberg, The role of osteocalcin in human glucose metabolism: marker or mediator? Nat. Rev. Endocrinol. 9(1), 43–55 (2013)
K. Kerschan-Schindl, M.M. Thalmann, E. Weiss, M. Tsironi, U. Foger-Samwald, J. Meinhart, K. Skenderi, P. Pietschmann, Changes in serum levels of myokines and Wnt-Antagonists after an ultramarathon race. PLoS One 10(7), e0132478 (2015)
K. Kerschan-Schindl, M. Thalmann, G.H. Sodeck, K. Skenderi, A.L. Matalas, S. Grampp, C. Ebner, P. Pietschmann, A 246-km continuous running race causes significant changes in bone metabolism. Bone 45(6), 1079–1083 (2009)
G. Lombardi, R. Corsetti, P. Lanteri, D. Grasso, E. Vianello, M.G. Marazzi, R. Graziani, A. Colombini, E. Galliera, M.M. Corsi Romanelli, G. Banfi, Reciprocal regulation of calcium-/phosphate-regulating hormones in cyclists during the Giro d’Italia 3-week stage race. Scand. J. Med. Sci. Sports 24(5), 779–787 (2014)
T. Hew-Butler, K.J. Stuempfle, M.D. Hoffman, Bone: an acute buffer of plasma sodium during exhaustive exercise? Horm. Metab. Res. 45(10), 697–700 (2013)
T. Bobbert, K. Mai, L. Brechtel, H.M. Schulte, B. Weger, A.F. Pfeiffer, J. Spranger, S. Diederich, Leptin and endocrine parameters in marathon runners. Int. J. Sports Med. 33(3), 244–248 (2012)
L.M. Burke, Nutrition strategies for the marathon: fuel for training and racing. Sports Med. 37(4-5), 344–347 (2007)
T. Montalcini, P. Gallotti, A. Coppola, V. Zambianchi, M. Fodaro, E. Galliera, M.G. Marazzi, S. Romeo, S. Giannini, M.M. Corsi Romanelli, A. Pujia, C. Gazzaruso, Association between low C-peptide and low lumbar bone mineral density in postmenopausal women without diabetes. Osteoporos. Int. 26(5), 1639–1646 (2015)
D. Hansen, R. Meeusen, A. Mullens, P. Dendale, Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity. Sports Med. 42(5), 415–431 (2012)
G. Banfi, A. Colombini, G. Lombardi, A. Lubkowska, Metabolic markers in sports medicine. Adv. Clin. Chem. 56, 1–54 (2012)
J. Pettus, I. Hirsch, S. Edelman, GLP-1 agonists in type 1 diabetes. Clin. Immunol. 149(3), 317–323 (2013)
T. Yada, B. Damdindorj, R.S. Rita, T. Kurashina, A. Ando, M. Taguchi, M. Koizumi, H. Sone, M. Nakata, M. Kakei, K. Dezaki, Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes Obes. Metab. 16(Suppl 1), 111–117 (2014)
G. Fernandez-Formoso, S. Perez-Sieira, D. Gonzalez-Touceda, C. Dieguez, S. Tovar, Leptin, 20 years of searching for glucose homeostasis. Life Sci. 140, 4–9 (2015)
M. Zaccaria, A. Ermolao, E. Brugin, M. Bergamin, Plasma leptin and energy expenditure during prolonged, moderate intensity, treadmill exercise. J. Endocrinol. Invest. 36(6), 396–401 (2013)
N.D. Roupas, I. Mamali, S. Maragkos, L. Leonidou, A.K. Armeni, G.K. Markantes, A. Tsekouras, G.C. Sakellaropoulos, K.B. Markou, N.A. Georgopoulos, The effect of prolonged aerobic exercise on serum adipokine levels during an ultra-marathon endurance race. Hormones (Athens) 12(2), 275–282 (2013)
G. Lombardi, A. Colombini, M. Freschi, R. Tavana, G. Banfi, Seasonal variation of bone turnover markers in top-level female skiers. Eur. J. Appl. Physiol. 111(3), 433–440 (2011)
G. Lombardi, G. Banfi, Effects of sample matrix and storage conditions on full-length visfatin measurement in blood. Clin. Chim. Acta 440, 140–142 (2015)
B. Lecka-Czernik, C.J. Rosen, Energy excess, glucose utilization and skeletal remodeling: new insights. J. Bone Miner. Res. 30(8), 1356–1361 (2015)
J. Upadhyay, O.M. Farr, C.S. Mantzoros, The role of leptin in regulating bone metabolism. Metabolism 64(1), 105–113 (2015)
P.J. Delhanty, B.C. van der Eerden, J.P. van Leeuwen, Ghrelin and bone. Biofactors 40(1), 41–48 (2014)
R. Corsetti, G. Lombardi, P. Lanteri, A. Colombini, R. Graziani, G. Banfi, Haematological and iron metabolism parameters in professional cyclists during the Giro d’Italia 3-weeks stage race. Clin. Chem. Lab. Med. 50(5), 949–956 (2012)
G. Banfi, G.S. Roi, A. Dolci, Erythrocytes, haemoglobin and packed cell volume in athletes performing races in altitude environment. Haematologica 85(E-letters), E12 (2000)
G. Banfi, G.S. Roi, A. Dolci, D. Susta, Behaviour of haematological parameters in athletes performing marathons and ultramarathons in altitude (‘skyrunners’). Clin. Lab. Haematol. 26(6), 373–377 (2004)
Acknowledgments
This work has been funded by the Italian Ministry of Health and the Italian Ministry of Education, University, and Research. The authors are indebted to Jincheng Xu, PhD, and Spyros Skafidas, MD, for their invaluable aid in performing and managing blood samplings. The authors would like to express their gratitude to Alberto Fondriest, Herbert Lorenzoni, Roberta Casagranda, and the Vigolana Trail® Organizing Committee. Finally, special thanks to the participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have conflict of interest. The results of the present study do not constitute endorsement by ACSM.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sansoni, V., Vernillo, G., Perego, S. et al. Bone turnover response is linked to both acute and established metabolic changes in ultra-marathon runners. Endocrine 56, 196–204 (2017). https://doi.org/10.1007/s12020-016-1012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1012-8