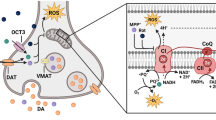
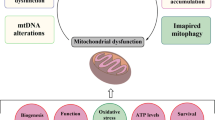
Abstract
As a key regulator of cell metabolism and survival, mechanistic target of rapamycin (mTOR) emerges as a novel therapeutic target for Parkinson’s disease (PD). A growing body of research indicates that restoring perturbed mTOR signaling in PD models can prevent neuronal cell death. Nevertheless, molecular mechanisms underlying mTOR-mediated effects in PD have not been fully understood yet. Here, we review recent progress in characterizing the association of mTOR signaling with PD risk factors and further discuss the potential roles of mTOR in PD.

Similar content being viewed by others
References
Anderson, G., & Maes, M. (2014). Neurodegeneration in Parkinson’s disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Molecular Neurobiology, 49, 771–783.
Anglade, P., et al. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and Histopathology, 12, 25–31.
Bao, X. Q., et al. (2012). FLZ protects dopaminergic neuron through activating protein kinase B/mammalian target of rapamycin pathway and inhibiting RTP801 expression in Parkinson’s disease models. Neuroscience, 202, 396–404.
Bjedov, I., et al. (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metabolism, 11, 35–46.
Bockaert, J., & Marin, P. (2015). mTOR in brain physiology and pathologies. Physiological Reviews, 95, 1157–1187.
Boland, D. F., & Stacy, M. (2012). The economic and quality of life burden associated with Parkinson’s disease: A focus on symptoms. The American Journal of Managed Care, 18, S168–S175.
Cannon, J. R., et al. (2013). Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson’s disease features and enhances vulnerability to mitochondrial impairment. Experimental Neurology, 240, 44–56.
Cartier, A. E., et al. (2012). Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS ONE, 7, e34713.
Chen, L., et al. (2008). MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. Journal of Neurochemistry, 105, 251–261.
Chen, L., et al. (2010). Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Laboratory Investigation, 90, 762–773.
Chen, L., et al. (2011). Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radical Biology & Medicine, 50, 624–632.
Chen, L. L., et al. (2014). Corynoxine, a natural autophagy enhancer, promotes the clearance of alpha-synuclein via Akt/mTOR pathway. Journal of Neuroimmune Pharmacology, 9, 380–387.
Cheung, Z. H., & Ip, N. Y. (2011). Autophagy deregulation in neurodegenerative diseases—Recent advances and future perspectives. Journal of Neurochemistry, 118, 317–325.
Choi, K. C., et al. (2010). A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. Journal of Neurochemistry, 112, 366–376.
Chong, Z. Z., et al. (2012). PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS ONE, 7, e45456.
Choo, A. Y., et al. (2008). Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proceedings of the National Academy of Sciences of the United States of America, 105, 17414–17419.
Chu, Y., et al. (2009). Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiology of Diseases, 35, 385–398.
Ciccone, S., et al. (2013). Parkinson’s disease: A complex interplay of mitochondrial DNA alterations and oxidative stress. International Journal of Molecular Sciences, 14, 2388–2409.
Cornu, M., Albert, V., & Hall, M. N. (2013). mTOR in aging, metabolism, and cancer. Current Opinion in Genetics & Development, 23, 53–62.
Crews, L., et al. (2010). Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE, 5, e9313.
Cuervo, A. M., et al. (2005). Autophagy and aging: The importance of maintaining “clean” cells. Autophagy, 1, 131–140.
Cullen, V., et al. (2011). Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Annals of Neurology, 69, 940–953.
Dauer, W., & Przedborski, S. (2003). Parkinson’s disease: Mechanisms and models. Neuron, 39, 889–909.
Dawson, T. M., & Dawson, V. L. (2003). Molecular pathways of neurodegeneration in Parkinson’s disease. Science, 302, 819–822.
Decressac, M., & Bjorklund, A. (2013). mTOR inhibition alleviates L-DOPA-induced dyskinesia in parkinsonian rats. Journal of Parkinsons Disease, 3, 13–17.
Decressac, M., et al. (2013). TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America, 110, E1817–E1826.
Dehay, B., et al. (2010). Pathogenic lysosomal depletion in Parkinson’s disease. Journal of Neuroscience, 30, 12535–12544.
Dehay, B., et al. (2015). Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurology, 14, 855–866.
Dennis, M. D., Kimball, S. R., & Jefferson, L. S. (2013). Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. Journal of Biological Chemistry, 288, 10–19.
Dexter, D. T., & Jenner, P. (2013). Parkinson disease: From pathology to molecular disease mechanisms. Free Radical Biology and Medicine, 62, 132–144.
DeYoung, M. P., et al. (2008). Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes & Development, 22, 239–251.
Dijkstra, A. A., et al. (2015). Evidence for immune response, axonal dysfunction and reduced endocytosis in the substantia nigra in early stage Parkinson’s disease. PLoS ONE, 10, e0128651.
Domanskyi, A., et al. (2011). Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB Journal, 25, 2898–2910.
Francois, A., et al. (2014). Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Molecular Brain, 7, 56.
Frias, M. A., et al. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Current Biology, 16, 1865–1870.
Gao, X., et al. (2002). Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nature Cell Biology, 4, 699–704.
Garcia-Martinez, J. M., & Alessi, D. R. (2008). mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochemical Journal, 416, 375–385.
Guertin, D. A., et al. (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Developmental Cell, 11, 859–871.
Gulhati, P., et al. (2011). mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Research, 71, 3246–3256.
Gumy, L. F., Tan, C. L., & Fawcett, J. W. (2010). The role of local protein synthesis and degradation in axon regeneration. Experimental Neurology, 223, 28–37.
Ha, J. Y., et al. (2014). Tnfaip8l1/Oxi-beta binds to FBXW5, increasing autophagy through activation of TSC2 in a Parkinson’s disease model. Journal of Neurochemistry, 129, 527–538.
Hara, T., et al. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441, 885–889.
He, C., et al. (2013). Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes, 62, 1270–1281.
Hu, Y., & Tong, Y. (2010). A trojan horse for Parkinson’s disease. Science Signaling, 3, pe13.
Huang, J., et al. (2008). The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Molecular and Cellular Biology, 28, 4104–4115.
Huang, J., et al. (2009). Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Research, 69, 6107–6114.
Hussain, S., et al. (2013). Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Molecular and Cellular Biology, 33, 1188–1197.
Imai, Y., et al. (2008). Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO Journal, 27, 2432–2443.
Inoki, K., Zhu, T., & Guan, K. L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115, 577–590.
Inoki, K., et al. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biology, 4, 648–657.
Jacinto, E., et al. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology, 6, 1122–1128.
Jaworski, J., & Sheng, M. (2006). The growing role of mTOR in neuronal development and plasticity. Molecular Neurobiology, 34, 205–219.
Jayaram, H. N., Kusumanchi, P., & Yalowitz, J. A. (2011). NMNAT expression and its relation to NAD metabolism. Current Medicinal Chemistry, 18, 1962–1972.
Jeong, J. K., et al. (2012). Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neuroscience Research, 73, 99–105.
Jiang, J., et al. (2013a). Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. International Journal of Molecular Medicine, 31, 825–832.
Jiang, T. F., et al. (2013b). Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. Journal of Neuroimmune Pharmacology, 8, 356–369.
Kahn, B. B., et al. (2005). AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism, 1, 15–25.
Kim, S. R., et al. (2011). Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Annals of Neurology, 70, 110–120.
Kim, H. J., et al. (2014a). Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells. Biomolecules & Therapeutics (Seoul), 22, 275–281.
Kim, K. A., et al. (2014b). High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biological and Pharmaceutical Bulletin, 37, 1248–1252.
Kim, C., et al. (2015). Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Reports, 13, 771–782.
Komatsu, M., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature, 441, 880–884.
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149, 274–293.
Lim, Y. M., et al. (2014). Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature Communications, 5, 4934.
Lin, X., et al. (2012). Conditional expression of Parkinson’s disease-related mutant a-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. Journal of Neuroscience, 32, 9248–9264.
Loewith, R., et al. (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular Cell, 10, 457–468.
Ma, X. M., & Blenis, J. (2009). Molecular mechanisms of mTOR-mediated translational control. Nature Reviews Molecular Cell Biology, 10, 307–318.
Magri, L., et al. (2011). Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell, 9, 447–462.
Maiese, K. (2015). Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Current Neurovascular Research, 12, 173–188.
Maiese, K., et al. (2010). Oxidative stress: Biomarkers and novel therapeutic pathways. Experimental Gerontology, 45, 217–234.
Maiese, K., et al. (2013). mTOR: On target for novel therapeutic strategies in the nervous system. Trends in Molecular Medicine, 19, 51–60.
Malagelada, C., Jin, Z. H., & Greene, L. A. (2008). RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. Journal of Neuroscience, 28, 14363–14371.
Malagelada, C., et al. (2006). RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. Journal of Neuroscience, 26, 9996–10005.
Malagelada, C., et al. (2010). Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. Journal of Neuroscience, 30, 1166–1175.
Manning, B. D., & Cantley, L. C. (2003). Rheb fills a GAP between TSC and TOR. Trends in Biochemical Sciences, 28, 573–576.
Menzies, F. M., Fleming, A., & Rubinsztein, D. C. (2015). Compromised autophagy and neurodegenerative diseases. Nature Reviews Neuroscience, 16, 345–357.
Murata, H., et al. (2011). A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: Activation of AKT via mTORC2. Journal of Biological Chemistry, 286, 7182–7189.
Mythri, R. B., et al. (2011). Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochemical Research, 36, 1452–1463.
Nakka, V. P., Prakash-Babu, P., & Vemuganti, R. (2016). Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: Potential therapeutic targets for acute CNS injuries. Molecular Neurobiology, 53, 532–544.
Nordstrom, U., et al. (2015). Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson’s disease. Neurobiology of Diseases, 73, 70–82.
Oh, W. J., & Jacinto, E. (2011). mTOR complex 2 signaling and functions. Cell Cycle, 10, 2305–2316.
Pan, T., et al. (2008). Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiology of Diseases, 32, 16–25.
Pan, T., et al. (2009). Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience, 164, 541–551.
Panov, A., et al. (2005). Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. Journal of Biological Chemistry, 280, 42026–42035.
Pearce, L. R., et al. (2011). Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochemical Journal, 436, 169–179.
Perez-Revuelta, B. I., et al. (2014). Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death and Disease, 5, e1209.
Perier, C., et al. (2003). The rotenone model of Parkinson’s disease. Trends in Neurosciences, 26, 345–346.
Perier, C., et al. (2005). Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proceedings of the National Academy of Sciences of the United States of America, 102, 19126–19131.
Peterson, T. R., et al. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell, 137, 873–886.
Ravikumar, B., et al. (2006). Rapamycin pre-treatment protects against apoptosis. Human Molecular Genetics, 15, 1209–1216.
Richardson, J. R., et al. (2005). Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicological Sciences, 88, 193–201.
Rieker, C., et al. (2011). Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. Journal of Neuroscience, 31, 453–460.
Rodriguez-Blanco, J., et al. (2012). Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. Journal of Neuroscience Research, 90, 1850–1860.
Romani-Aumedes, J., et al. (2014). Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death and Disease, 5, e1364.
Ruffels, J., Griffin, M., & Dickenson, J. M. (2004). Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. European Journal of Pharmacology, 483, 163–173.
Santini, E., et al. (2009). Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Science Signaling, 2, 36.
Sarbassov, D. D., et al. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current Biology, 14, 1296–1302.
Sarbassov, D. D., et al. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell, 22, 159–168.
Sarkar, S., et al. (2005). Lithium induces autophagy by inhibiting inositol monophosphatase. Journal of Cell Biology, 170, 1101–1111.
Sarkar, S., et al. (2007). Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. Journal of Biological Chemistry, 282, 5641–5652.
Schapira, A. H., et al. (2014). Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet, 384, 545–555.
Selvaraj, S., et al. (2012). Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. The Journal of Clinical Investigation, 122, 1354–1367.
Shang, Y. C., et al. (2011). Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Current Neurovascular Research, 8, 270–285.
Shang, Y. C., et al. (2012a). Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging, 4, 187–201.
Shang, Y. C., et al. (2012b). Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Current Neurovascular Research, 9, 239–249.
Shulman, J. M., De Jager, P. L., & Feany, M. B. (2011). Parkinson’s disease: Genetics and pathogenesis. Annual Review of Pathology: Mechanisms of Disease, 6, 193–222.
Silva, D. F., et al. (2011). Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Current Alzheimer Research, 8, 563–572.
Spencer, B., et al. (2009). Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. Journal of Neuroscience, 29, 13578–13588.
Subramaniam, S., et al. (2012). Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nature Neuroscience, 15, 191–193.
Swiech, L., et al. (2008). Role of mTOR in physiology and pathology of the nervous system. Biochimica et Biophysica Acta, 1784, 116–132.
Tain, L. S., et al. (2009). Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nature Neuroscience, 12, 1129–1135.
Tee, A. R., et al. (2002). Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proceedings of the National Academy of Sciences of the United States of America, 99, 13571–13576.
Thoreen, C. C., & Sabatini, D. M. (2009). Rapamycin inhibits mTORC1, but not completely. Autophagy, 5, 725–726.
Thoreen, C. C., et al. (2009). An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. Journal of Biological Chemistry, 284, 8023–8032.
Tieu, K. (2011). A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine, 1, a009316.
Vakifahmetoglu-Norberg, H., Xia, H. G., & Yuan, J. (2015). Pharmacologic agents targeting autophagy. Journal of Clinical Investigation, 125, 5–13.
Vila, M., et al. (2011). Lysosomal membrane permeabilization in Parkinson disease. Autophagy, 7, 98–100.
Wang, H., et al. (2012a). Proline-rich Akt substrate of 40 kDa (PRAS40): A novel downstream target of PI3 k/Akt signaling pathway. Cellular Signalling, 24, 17–24.
Wang, Y., et al. (2012b). Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. American Journal of Translational Research, 4, 44–51.
Webb, J. L., et al. (2003). Alpha-synuclein is degraded by both autophagy and the proteasome. Journal of Biological Chemistry, 278, 25009–25013.
Williams, A. C., et al. (2012). Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: Hypotheses and lessons from pellagra. Current Gerontology and Geriatrics Research, 2012, 302875.
Wills, J., et al. (2012). Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE, 7, e30745.
Wong, E., & Cuervo, A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nature Neuroscience, 13, 805–811.
Wu, A. G., et al. (2013). Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant a-synuclein and huntingtin in PC-12 cells. International Journal of Molecular Sciences, 14, 22618–22641.
Wullschleger, S., Loewith, R., & Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell, 124, 471–484.
Xiong, X., et al. (2014). PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiology of Diseases, 66, 43–52.
Xu, B., et al. (2011). Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE, 6, e19052.
Xu, Y., et al. (2014). Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cellular Signalling, 26, 1680–1689.
Yacoubian, T. A., & Standaert, D. G. (2009). Targets for neuroprotection in Parkinson’s disease. Biochimica et Biophysica Acta, 1792, 676–687.
Yamada, E., & Singh, R. (2012). Mapping autophagy on to your metabolic radar. Diabetes, 61, 272–280.
Yan, J. Q., et al. (2014). Overexpression of human E46K mutant a-synuclein impairs macroautophagy via inactivation of JNK1-Bcl-2 pathway. Molecular Neurobiology, 50, 685–701.
Yang, H., et al. (2011). Oxidative stress and diabetes mellitus. Clinical Chemistry and Laboratory Medicine, 49, 1773–1782.
Yu, W. H., et al. (2009). Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. American Journal of Pathology, 175, 736–747.
Zeng, X. S., et al. (2014). The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radical Biology and Medicine, 67, 10–18.
Zhang, Z., et al. (2015). Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radical Biology and Medicine, 84, 331–343.
Zhou, Q., et al. (2015). Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicological Sciences, 143, 81–96.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 11375213, 21390411) and Hundred Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lan, Ap., Chen, J., Zhao, Y. et al. mTOR Signaling in Parkinson’s Disease. Neuromol Med 19, 1–10 (2017). https://doi.org/10.1007/s12017-016-8417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8417-7