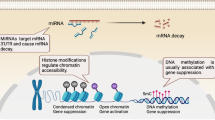
Abstract
B lymphocytes are generally recognized as the essential component of humoral immunity and also a regulator of innate immunity. The development of B cells is precisely regulated by a variety of factors via different mechanisms, including cytokine/cytokine receptors, signal transduction molecules, and transcription factors. Recent findings suggest that epigenetic factors, such as DNA methylation, histone modification, and non-coding RNA, play critical roles in establishing B cell lineage–specific gene expression profiles to define and sustain B cell identity and function. Epigenetic modifications are also sensitive to external stimuli and might bridge genetic and environmental factors in the pathogenesis or control of B-cell-related immune disorders, such as autoimmune diseases, lymphoma, and leukemia. Better understanding of the epigenetic mechanisms for regulating B cell development and involving B cell abnormal differentiation and function will shed light on the design of new therapeutic approaches to B-cell-related diseases, and potential candidates of epigenetic modulators may be identified to target epigenetic pathways to prevent or treat B cell disorders. We summarize the relevance of epigenetic marks and landscapes in the stages of B cell development, discuss the interaction of the transcriptional networks and epigenetic changes, and review the involvement of epigenetic risk in the pathogenesis of B-cell-related diseases. Understanding how specific epigenetic alterations contribute to the development of B-cell-related autoimmunity and malignancies is instrumental to control B cell disorders.

Similar content being viewed by others
References
Clark MR, Mandal M, Ochiai K, Singh H (2014) Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol 14:69–80
Bao Y, Cao X (2014) The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review. J Autoimmun 55:10–23
Faurschou M, Jayne DR (2014) Anti-B cell antibody therapies for inflammatory rheumatic diseases. Annu Rev Med 65:263–278
Yu B, Mao Y, Bai LY, Herman SE et al (2013) Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood 121:136–147
Cooper MD (2015) The early history of B cells. Nat Rev Immunol 15:191–197
Reth M, Nielsen P (2014) Signaling circuits in early B-cell development. Adv Immunol 122:129–175
De Silva NS, Klein U (2015) Dynamics of B cells in germinal centres. Nat Rev Immunol 15:137–148
Li G, Zan H, Xu Z, Casali P (2013) Epigenetics of the antibody response. Trends Immunol 34:460–470
Choukrallah MA, Matthias P (2014) The interplay between chromatin and transcription factor networks during B cell development: who pulls the trigger first? Front Immunol 5:156
Medvedovic J, Ebert A, Tagoh H, Busslinger M (2011) Pax5: a master regulator of B cell development and leukemogenesis. Adv Immunol 111:179–206
Mercer EM, Lin YC, Benner C et al (2011) Multilineage priming of enhancer repertoires precedes commitment to the B and myeloid cell lineages in hematopoietic progenitors. Immunity 35:413–425
Heinz S, Benner C, Spann N et al (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589
Trowbridge JJ, Snow JW, Kim J, Orkin SH (2009) DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell 5:442–449
Bröske AM, Vockentanz L, Kharazi S et al (2009) DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet 41:1207–1215
Challen GA, Sun D, Jeong M et al (2011) Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 44:23–31
Kong KY, Owens KS, Rogers JH et al (2010) MIR-23A microRNA cluster inhibits B-cell development. Exp Hematol 38:629–640, e1
Maier H, Ostraat R, Gao H et al (2004) Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immunol 5:1069–1077
Gao H, Lukin K, Ramírez J, Fields S, Lopez D, Hagman J (2009) Opposing effects of SWI/SNF and Mi-2/NuRD chromatin remodeling complexes on epigenetic reprogramming by EBF and Pax5. Proc Natl Acad Sci U S A 106:11258–11263
Zouali M (2013) The epigenetic landscape of B lymphocyte tolerance to self. FEBS Lett 587:2067–2073
Cho YW, Hong T, Hong S et al (2007) PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem 282:20395–20406
McManus S, Ebert A, Salvagiotto G et al (2011) The transcription factor Pax5 regulates its target genes by recruiting chromatin-modifying proteins in committed B cells. EMBO J 30:2388–2404
Hatta M, Cirillo LA (2007) Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J Biol Chem 282:35583–35593
Lin YC, Jhunjhunwala S, Benner C et al (2010) A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol 11:635–643
Kuchen S, Resch W, Yamane A et al (2010) Regulation of microRNA expression and abundance during lymphopoiesis. Immunity 32:828–839
O’Carroll D, Mecklenbrauker I, Das PP et al (2007) A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev 21:1999–2004
Koralov SB, Muljo SA, Galler GR et al (2008) Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132:860–874
Xiao C, Srinivasan L, Calado DP et al (2008) Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 9:405–414
Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF (2007) miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A 104:7080–7085
Xiao C, Calado DP, Galler G et al (2007) MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131:146–159
Rao DS, O’Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D (2010) MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity 33:48–59
Chang TC, Wentzel EA, Kent OA et al (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26:745–752
Raver-Shapira N, Marciano E, Meiri E et al (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743
Nakase H, Takahama Y, Akamatsu Y (2003) Effect of CpG methylation on RAG1/RAG2 reactivity: implications of direct and indirect mechanisms for controlling V(D)J cleavage. EMBO Rep 4:774–780
Selimyan R, Gerstein RM, Ivanova I et al (2013) Localized DNA demethylation at recombination intermediates during immunoglobulin heavy chain gene assembly. PLoS Biol 11:e1001475
Cherry SR, Beard C, Jaenisch R, Baltimore D (2000) V(D)J recombination is not activated by demethylation of the kappa locus. Proc Natl Acad Sci U S A 97:8467–8472
Xu CR, Feeney AJ (2009) The epigenetic profile of Ig genes is dynamically regulated during B cell differentiation and is modulated by pre-B cell receptor signaling. J Immunol 182:1362–1369
Walter K, Bonifer C, Tagoh H (2008) Stem cell-specific epigenetic priming and B cell-specific transcriptional activation at the mouse Cd19 locus. Blood 112:1673–1682
Knoll M, Simmons S, Bouquet C, Grün JR, Melchers F (2013) miR-221 redirects precursor B cells to the BM and regulates their residence. Eur J Immunol 43:2497–2506
Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K (2004) Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell 117:787–800
Shaffer AL, Lin KI, Kuo TC et al (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17:51–62
Nutt SL, Taubenheim N, Hasbold J, Corcoran LM, Hodgkin PD (2011) The genetic network controlling plasma cell differentiation. Semin Immunol 23:341–349
Muto A, Ochiai K, Kimura Y et al (2010) Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J 29:4048–4061
Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM (1997) Control of inflammation, cytokine expression andgerminal center formation by BCL-6. Science 276:589–592
Ye BH, Cattoretti G, Shen Q et al (1997) The BCL-6 proto-oncogene controlsgerminal-centre formation and Th2-type inflammation. Nat Genet 16:161–170
Reljic R, Wagner SD, Peakman LJ, Fearon DT (2000) Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 192:1841–1848
Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM (2000) BCL-6 represses genes that function in lymphocyte differentiation, inflammation and cell cycle control. Immunity 13:199–212
Fujita N, Jaye DL, Geigerman C et al (2004) MTA3 and theMi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 119:75–86
Fujita N, Jaye DL, Kajita M et al (2003) MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113:207–219
Batlle-López A, Cortiguera MG, Rosa-Garrido M et al (2015) Novel CTCF binding at a site in exon1A of BCL6 is associated with active histone marks and a transcriptionally active locus. Oncogene 34:246–256
Ramachandrareddy H, Bouska A, Shen Y et al (2010) BCL6 promoter interacts with far upstream sequences with greatly enhanced activating histone modifications in germinal center B cells. Proc Natl Acad Sci U S A 107:11930–11935
Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K (2000) Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol 20:2592–2603
Ren B, Chee KJ, Kim TH, Maniatis T (1999) PRDI-BF1/Blimp-1 repression is mediated by corepressors of the Groucho family of proteins. Genes Dev 13:125–137
Gyory I, Wu J, Fejér G, Seto E, Wright KL (2004) PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol 5:299–308
Ancelin K, Lange UC, Hajkova P et al (2006) Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol 8:623–630
Su ST, Ying HY, Chiu YK, Lin FR, Chen MY, Lin KI (2009) Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol Cell Biol 29:1421–1431
Ying HY, Su ST, Hsu PH et al (2012) SUMOylation of Blimp-1 is critical for plasma cell differentiation. EMBO Rep 13:631–637
Belver L, de Yébenes VG, Ramiro AR (2010) MicroRNAs prevent the generation of autoreactive antibodies. Immunity 33:713–722
Thai TH, Calado DP, Casola S et al (2007) Regulation of the germinal center response by microRNA-155. Science 316:604–608
Rodriguez A, Vigorito E, Clare S et al (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611
Vigorito E, Perks KL, Abreu-Goodger C et al (2007) microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 27:847–859
Dorsett Y, McBride KM, Jankovic M et al (2008) MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 28(5):630–638
Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, Papavasiliou FN. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity 28:621–629
Shlomchik MJ (2008) Sites and stages of autoreactive B cell activation and regulation. Immunity 28:18–28
Vinuesa CG, Sanz I, Cook MC (2009) Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9:845–857
Hertz M, Nemazee D (1997) BCR ligation induces receptor editing in IgM + IgD- bone marrow B cells in vitro. Immunity 6:429–436
Melamed D, Nemazee D (1997) Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc Natl Acad Sci U S A 94:9267–9272
Mazari L, Ouarzane M, Zouali M (2007) Subversion of B lymphocyte tolerance by hydralazine, a potential mechanism for drug-induced lupus. Proc Natl Acad Sci U S A 104:6317–6322
Gupta B, Hawkins RD (2015) Epigenomics of autoimmune diseases. Immunol Cell Biol 93:271–276
Garaud S, Le Dantec C, Jousse-Joulin S et al (2009) IL-6 modulates CD5 expression in B cells from patients with lupus by regulating DNA methylation. J Immunol 182:5623–5632
Forster N, Gallinat S, Jablonska J, Weiss S, Elsässer HP, Lutz W (2007) p300 protein acetyltransferase activity suppresses systemic lupus erythematosus-like autoimmune disease in mice. J Immunol 178:6941–6948
Thai TH, Patterson HC, Pham DH, Kis-Toth K, Kaminski DA, Tsokos GC (2013) Deletion of microRNA-155 reduces autoantibody responses and alleviates lupus-like disease in the Fas(lpr) mouse. Proc Natl Acad Sci U S A 110:20194–20199
Wang YZ, Tian FF, Yan M et al (2014) Delivery of an miR155 inhibitor by anti-CD20 single-chain antibody into B cells reduces the acetylcholine receptor-specific autoantibodies and ameliorates experimental autoimmune myasthenia gravis. Clin Exp Immunol 176:207–221
Zhang J, Jia G, Liu Q et al (2015) Silencing miR-146a influences B cells and ameliorates experimental autoimmune myasthenia gravis. Immunology 144:56–67
Mok Y, Schwierzeck V, Thomas DC et al (2013) MiR-210 is induced by Oct-2, regulates B cells, and inhibits autoantibody production. J Immunol 191:3037–3048
Stagakis E, Bertsias G, Verginis P et al (2011) Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann Rheum Dis 70:1496–1506
Pan W, Zhu S, Yuan M et al (2010) MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 184:6773–6781
Garchow BG, Bartulos Encinas O, Leung YT et al (2011) Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med 3:605–615
Yuan Y, Kasar S, Underbayev C et al (2012) Role of microRNA-15a in autoantibody production in interferon-augmented murine model of lupus. Mol Immunol 52:61–70
Liu Y, Dong J, Mu R et al (2013) MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn. Arthritis Rheum 65:1603–1611
Nakasa T, Miyaki S, Okubo A et al (2008) Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 58:1284–1292
Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC (2011) Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers 30:171–179
Klein U, Dalla-Favera R (2008) Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 8:22–33
Chapman MA, Lawrence MS, Keats JJ et al (2011) Initial genome sequencing and analysis of multiple myeloma. Nature 471:467–472
Morin RD, Mendez-Lago M, Mungall AJ et al (2011) Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476:298–303
Velichutina I, Shaknovich R, Geng H et al (2010) EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood 116:5247–5255
Raaphorst FM, van Kemenade FJ, Fieret E et al (2000) Cutting edge: polycomb gene expression patterns reflect distinct B cell differentiation stages in human germinal centers. J Immunol 164:1–4
van Kemenade FJ, Raaphorst FM, Blokzijl T et al (2001) Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood 97:3896–3901
Morin RD, Johnson NA, Severson TM et al (2010) Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42:181–185
Bödör C, O’Riain C, Wrench D et al (2011) EZH2 Y641 mutations in follicular lymphoma. Leukemia 25:726–729
Caganova M, Carrisi C, Varano G et al (2013) Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest 123:5009–5022
Beguelin W, Popovic R, Teater M et al (2013) EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23:677–692
McCabe MT, Ott HM, Ganji G et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492:108–112
Knutson SK, Wigle TJ, Warholic NM et al (2012) A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol 8:890–896
Pasqualucci L, Dominguez-Sola D, Chiarenza A et al (2011) Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 471:189–195
Cerchietti LC, Hatzi K, Caldas-Lopes E et al (2010) BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest pii: 42869. doi: 10.1172/JCI42869. [Epub ahead of print]
Cerchietti LC, Yang SN, Shaknovich R et al (2009) A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood 113:3397–3405
Giulino-Roth L, Wang K, MacDonald TY et al (2012) Targeted genomic sequencing of pediatric Burkitt lymphoma identifies recurrent alterations in antiapoptotic and chromatin-remodeling genes. Blood 120:5181–5184
Quesada V, Conde L, Villamor N et al (2011) Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet 44:47–52
Zang ZJ, Cutcutache I, Poon SL et al (2012) Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet 44:570–574
Guan B, Wang TL, Shih IM (2011) ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res 71:6718–6127
Harada A, Okada S, Konno D et al (2012) Chd2 interacts with H3.3 to determine myogenic cell fate. EMBO J 31:2994–3007
Nagarajan P, Onami TM, Rajagopalan S, Kania S, Donnell R, Venkatachalam S (2009) Role of chromodomain helicase DNA-binding protein 2 in DNA damage response signaling and tumorigenesis. Oncogene 28:1053–1062
Calin GA, Dumitru CD, Shimizu M et al (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 99:15524–15529
Döhner H, Stilgenbauer S, Benner A et al (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343:1910–1916
Rawstron AC, Bennett FL, O’Connor SJ et al (2008) Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 359:575–583
Avet-Loiseau H, Li JY, Morineau N et al (1999) Monosomy 13 is associated with the transition of monoclonal gammopathy of undetermined significance to multiple myeloma. Intergroupe Francophone du Myelome. Blood 94:2583–2589
Klein U, Lia M, Crespo M et al (2010) The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17:28–40
Cimmino A, Calin GA, Fabbri M et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102:13944–13949
He L, Thomson JM, Hemann MT et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833
Costinean S, Zanesi N, Pekarsky Y et al (2006) Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A 103:7024–7029
Basso K, Schneider C, Shen Q et al (2012) BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med 209:2455–2465
Kluiver J, Haralambieva E, de Jong D et al (2006) Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosom Cancer 45:147–153
Kluiver J, Poppema S, de Jong D et al (2005) BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol 207:243–249
Eis PS, Tam W, Sun L et al (2005) Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A 102:3627–3632
Olive V, Bennett MJ, Walker JC et al (2009) miR-19 is a key oncogenic component of mir-17-92. Genes Dev 23:2839–2849
Mi S, Li Z, Chen P et al (2010) Aberrant overexpression and function of the miR-17-92 cluster in MLL-rearranged acute leukemia. Proc Natl Acad Sci U S A 107:3710–371
Mu P, Han YC, Betel D et al (2009) Genetic dissection of the miR-17 ∼ 92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev 23:2806–2811
Van Haaften G, Agami R (2010) Tumorigenicity of the miR-17-92 cluster distilled. Genes Dev 24:1–4
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (81172805), the Special Scientific Research Fund of Health Public Welfare Profession of China (201302018, 201202019), and Shanghai Rising-Star Program (14QA1404900).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bao, Y., Cao, X. Epigenetic Control of B Cell Development and B-Cell-Related Immune Disorders. Clinic Rev Allerg Immunol 50, 301–311 (2016). https://doi.org/10.1007/s12016-015-8494-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8494-7