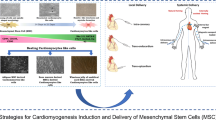
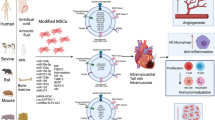
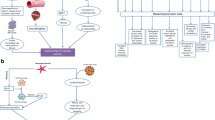
Abstract
Cardiovascular disorders (CVDs) are the leading cause of global death, widely occurs due to irreparable loss of the functional cardiomyocytes. Stem cell-based therapeutic approaches, particularly the use of Mesenchymal Stem Cells (MSCs) is an emerging strategy to regenerate myocardium and thereby improving the cardiac function after myocardial infarction (MI). Most of the current approaches often employ the use of various biological and chemical factors as cues to trigger and modulate the differentiation of MSCs into the cardiac lineage. However, the recent advanced methods of using specific epigenetic modifiers and exosomes to manipulate the epigenome and molecular pathways of MSCs to modify the cardiac gene expression yield better profiled cardiomyocyte like cells in vitro. Hitherto, the role of cardiac specific inducers triggering cardiac differentiation at the cellular and molecular level is not well understood. Therefore, the current review highlights the impact and recent trends in employing biological and chemical inducers on cardiac differentiation of MSCs. Thereby, deciphering the interactions between the cellular microenvironment and the cardiac inducers will help us to understand cardiomyogenesis of MSCs. Additionally, the review also provides an insight on skeptical roles of the cell free biological factors and extracellular scaffold assisted mode for manipulation of native and transplanted stem cells towards translational cardiac research.
Graphical Abstract





Similar content being viewed by others
Data Availability
Not applicable.
Change history
25 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12015-021-10183-1
Abbreviations
- 5-aza:
-
5-Azacytidine
- AKT:
-
protein kinase B
- bFGF:
-
Basic fibroblast growth factor
- BMPs:
-
Bone morphogenetic proteins
- BM-MSCs:
-
Bone marrow-derived MSCs
- cTnI:
-
Cardiac troponin I
- cTnT:
-
Cardiac troponin T
- CX-43:
-
Connexin43
- CVDs:
-
Cardiovascular disorders
- DMSO:
-
Dimethyl sulfoxide
- DNMTs:
-
DNA methyltransferase
- Dll-1:
-
Delta-like 1
- DZNep:
-
3-Deazaneplanocin
- ECM:
-
Extracellular matrix
- ESCs:
-
Embryonic stem cells
- FBS:
-
Fetal Bovine Serum
- GATA4:
-
GATA binding protein4
- H3K27me3:
-
Histone 3 lysine 27 trimethylaion
- HAT:
-
Histone acetyltransferase
- Hand2:
-
Heart and Neural Crest DerivativesExpressed 2
- HGF:
-
Hepatocyte growth factor
- HDAC:
-
Histone deacetylase
- HMTs:
-
Histone methyltransferases
- IGF-1:
-
Insulin-like growth factor-1
- iPSCs:
-
Induced pluripotent stem cells
- KLF4:
-
Kruppel-like factor 2
- Mef2c:
-
Myocyte-specific enhancerfactor 2C
- MHC:
-
Myosin heavy chain
- miRNAs:
-
Microribonucleic acids
- MI:
-
Myocardialinfarction
- MSCs:
-
Mesenchymal stem cells
- MYH6:
-
Myosin heavy chain 6
- MYL7:
-
Myosin light chain 7
- Nkx2.5:
-
Homeobox protein 2.5
- PCL:
-
Poly(ε-caprolactone)
- SAHA:
-
Suberoylanilide hydroxamic acid
- SDF1:
-
Stromal cell-derived factor1
- TGFβs:
-
Transforming growth factor betas
- TBX5:
-
T-box transcription factor
- Trichostatin A:
-
TSA
- VEGF:
-
Vascular endothelial growth factor
References
Jagannathan, R., Patel, S. A., Ali, M. K., & Narayan, K. M. V. (2019). Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Current Diabetes Reports, 19(7), 44. https://doi.org/10.1007/s11892-019-1161-2.
Roth, G. A., Johnson, C., Abajobir, A., Abd-Allah, F., Abera, S. F., Abyu, G., Ahmed, M., Aksut, B., Alam, T., Alam, K., Alla, F., Alvis-Guzman, N., Amrock, S., Ansari, H., Ärnlöv, J., Asayesh, H., Atey, T. M., Avila-Burgos, L., Awasthi, A., … Murray, C. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology, 70(1), 1–25. https://doi.org/10.1016/j.jacc.2017.04.052.
Hare, J. M., & Chaparro, S. V. (2008). Cardiac regeneration and stem cell therapy. Current Opinion in Organ Transplantation, 13(5), 536–542.
Pendyala, L., Goodchild, T., Gadesam, R. R., Chen, J., Robinson, K., Chronos, N., & Hou, D. (2008). Cellular cardiomyoplasty and cardiac regeneration. Current Cardiology Reviews, 4(2), 72–80. https://doi.org/10.2174/157340308784245748.
Eisenberg, L. M., Kubalak, S. W., & Eisenberg, C. A. (2004). Stem cells and the formation of the myocardium in the vertebrate embryo. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 276(1), 2–12. https://doi.org/10.1002/ar.a.10130.
Liu, Z., Naveed, M., Baig, M. M. F. A., Mikrani, R., Li, C., Saeed, M., Zhang, Q., Farooq, M. A., Zubair, H. M., & Xiaohui, Z. (2021). Therapeutic approach for global myocardial injury using bone marrow-derived mesenchymal stem cells by cardiac support device in rats. Biomedical Microdevices, 23(1), 5. https://doi.org/10.1007/s10544-020-00538-9.
Pittenger, M. F., & Martin, B. J. (2004). Mesenchymal stem cells and their potential as cardiac therapeutics. Circulation Research, 95(1), 9–20. https://doi.org/10.1161/01.RES.0000135902.99383.6f.
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells - current trends and future prospective. Bioscience Reports, 35(2). https://doi.org/10.1042/BSR20150025.
Fukuda, K. (2003). Use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplantation, 32(Suppl 1), 25–27. https://doi.org/10.1038/sj.bmt.1703940.
Yang, G., Xiao, Z., Ren, X., Long, H., Ma, K., Qian, H., & Guo, Y. (2017). Obtaining spontaneously beating cardiomyocyte-like cells from adipose-derived stromal vascular fractions cultured on enzyme-crosslinked gelatin hydrogels. Scientific Reports, 7(1), 41781. https://doi.org/10.1038/srep41781.
Qian, Q., Qian, H., Zhang, X., Zhu, W., Yan, Y., Ye, S., Peng, X., Li, W., Xu, Z., Sun, L., & Xu, W. (2012). 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells and Development, 21(1), 67–75. https://doi.org/10.1089/scd.2010.0519.
Jiang, S., & Zhang, S. (2017). Differentiation of cardiomyocytes from amniotic fluidderived mesenchymal stem cells by combined induction with transforming growth factor beta1 and 5azacytidine. Molecular Medicine Reports, 16(5), 5887–5893. https://doi.org/10.3892/mmr.2017.7373.
Avgustinova, A., & Benitah, S. A. (2016). Epigenetic control of adult stem cell function. Nature Reviews. Molecular Cell Biology, 17(10), 643–658. https://doi.org/10.1038/nrm.2016.76.
Maalouf, W. E., Liu, Z., Brochard, V., Renard, J.-P., Debey, P., Beaujean, N., & Zink, D. (2009). Trichostatin A treatment of cloned mouse embryos improves constitutive heterochromatin remodeling as well as developmental potential to term. BMC Developmental Biology, 9, 11. https://doi.org/10.1186/1471-213X-9-11.
Shi, S., Wu, X., Wang, X., Hao, W., Miao, H., Zhen, L., & Nie, S. (2016). Differentiation of bone marrow mesenchymal stem cells to cardiomyocyte-like cells is regulated by the combined low dose treatment of transforming growth factor-β1 and 5-azacytidine. Stem Cells International, 2016, 3816256. https://doi.org/10.1155/2016/3816256.
Lan, H., Xue, Q., Liu, Y., Jin, K., Fang, X., & Shao, H. (2021). The emerging therapeutic role of mesenchymal stem cells in anthracycline-induced cardiotoxicity. Cell and Tissue Research. https://doi.org/10.1007/s00441-020-03364-w.
Golpanian, S., Wolf, A., Hatzistergos, K. E., & Hare, J. M. (2016). Rebuilding the damaged heart: Mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiological Reviews, 96(3), 1127–1168. https://doi.org/10.1152/physrev.00019.2015.
Varderidou-Minasian, S., & Lorenowicz, M. J. (2020). Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics, 10(13), 5979–5997. https://doi.org/10.7150/thno.40122.
Beigi, F., Schmeckpeper, J., Pow-Anpongkul, P., Payne, J. A., Zhang, L., Zhang, Z., Huang, J., Mirotsou, M., & Dzau, V. J. (2013). C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the PI3K-AKT-CDK7 pathway. Circulation Research, 113(4), 372–380. https://doi.org/10.1161/CIRCRESAHA.113.301075.
Zhang, R., Liu, Y., Yan, K., Chen, L., Chen, X.-R., Li, P., Chen, F.-F., & Jiang, X.-D. (2013). Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. Journal of Neuroinflammation, 10, 1–12.
Hare, J. M., Fishman, J. E.,Gerstenblith, G., DiFede Velazquez, D. L., Zambrano, J. P., Suncion, V. Y.,Tracy, M., Ghersin, E., Johnston, P. V, Brinker, J. A., Breton, E.,Davis-Sproul, J., Schulman, I. H., Byrnes, J., Mendizabal, A. M., Lowery, M.H., Rouy, D., Altman, P., Wong Po Foo, C., … Lardo, A. (2012). Comparison ofallogeneic vs autologous bone marrow-derived mesenchymal stem cells deliveredby transendocardial injection in patients with ischemic cardiomyopathy: thePOSEIDON randomized trial. JAMA, 308(22), 2369–2379. https://doi.org/10.1001/jama.2012.25321.
Heldman, A. W., DiFede, D. L.,Fishman, J. E., Zambrano, J. P., Trachtenberg, B. H., Karantalis, V., Mushtaq,M., Williams, A. R., Suncion, V. Y., McNiece, I. K., Ghersin, E., Soto, V.,Lopera, G., Miki, R., Willens, H., Hendel, R., Mitrani, R., Pattany, P.,Feigenbaum, G., … Hare, J. M. (2014). Transendocardial mesenchymal stem cells andmononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFTrandomized trial. JAMA - Journal of the American Medical Association, 311(1), 62–73. https://doi.org/10.1001/jama.2013.282909.
Y., S. V., Eduard, G., E., F.J., Pablo, Z. J., Vasileios, K., Nicole, M., H., N. K., Gary, G., L., D. V. D.,Elayne, B., Kranthi, S., H., S. I., N., T. S., R., W. A., Cristina, S., V., J.P., Jeffrey, B., Peter, A., Muzammil, M., … W., H. A. (2014). Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? Circulation Research, 114(8), 1292–1301. https://doi.org/10.1161/CIRCRESAHA.114.302854.
Hare, J. M., DiFede, D. L., Rieger, A. C., Florea, V., Landin, A. M., El-Khorazaty, J., Khan, A., Mushtaq, M., Lowery, M. H., Byrnes, J. J., Hendel, R. C., Cohen, M. G., Alfonso, C. E., Valasaki, K., Pujol, M. V., Golpanian, S., Ghersin, E., Fishman, J. E., Pattany, P., … Heldman, A. W. (2017). Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy. Journal of the American College of Cardiology, 69(5), 526–537. https://doi.org/10.1016/j.jacc.2016.11.009.
Potapova, I. A., Doronin, S. V., Kelly, D. J., Rosen, A. B., Schuldt, A. J. T., Lu, Z., Kochupura, P. V., Robinson, R. B., Rosen, M. R., Brink, P. R., Gaudette, G. R., & Cohen, I. S. (2008). Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. American Journal of Physiology - Heart and Circulatory Physiology, 295(6), 2257–2263. https://doi.org/10.1152/ajpheart.00219.2008.
Kim, S. H., Cho, J. H., Lee, Y. H., Lee, J. H., Kim, S. S., Kim, M. Y., Lee, M. G., Kang, W. Y., Lee, K. S., Ahn, Y. K., Jeong, M. H., & Kim, H. S. (2018). Improvement in Left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall ST-segment elevation myocardial infarction. Cardiovascular Drugs and Therapy, 32(4), 329–338. https://doi.org/10.1007/s10557-018-6804-z.
Premer, C., Blum, A., Bellio, M. A., Schulman, I. H., Hurwitz, B. E., Parker, M., Dermarkarian, C. R., DiFede, D. L., Balkan, W., Khan, A., & Hare, J. M. (2015). Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine, 2(5), 467–475. https://doi.org/10.1016/j.ebiom.2015.03.020.
Hatzistergos, K. E., Saur, D., Seidler, B., Balkan, W., Breton, M., Valasaki, K., Takeuchi, L. M., Landin, A. M., Khan, A., & Hare, J. M. (2016). Stimulatory effects of mesenchymal stem cells on cKit + cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circulation Research, 119(8), 921–930. https://doi.org/10.1161/CIRCRESAHA.116.309281.
Chung, E. S., Miller, L., Patel, A. N., Anderson, R. D., Mendelsohn, F. O., Traverse, J., Silver, K. H., Shin, J., Ewald, G., Farr, M. J., Anwaruddin, S., Plat, F., Fisher, S. J., AuWerter, A. T., Pastore, J. M., Aras, R., & Penn, M. S. (2015). Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: The STOP-HF randomized Phase II trial. European Heart Journal, 36(33), 2228–2238. https://doi.org/10.1093/eurheartj/ehv254.
Prakash Parthiban, S., Rana, D., Jabbari, E., Benkirane-Jessel, N., & Ramalingam, M. (2017). Covalently immobilized VEGF-mimicking peptide with gelatin methacrylate enhances microvascularization of endothelial cells. Acta Biomaterialia, 51, 330–340. https://doi.org/10.1016/j.actbio.2017.01.046.
Nakanishi, C., Nagaya, N., Ohnishi, S., Yamahara, K., Takabatake, S., Konno, T., Hayashi, K., Kawashiri, M.-A., Tsubokawa, T., & Yamagishi, M. (2011). Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circulation Journal: Official Journal of the Japanese Circulation Society, 75(9), 2260–2268. https://doi.org/10.1253/circj.cj-11-0246.
Mylotte, L. A., Duffy, A. M., Murphy, M., O’Brien, T., Samali, A., Barry, F., & Szegezdi, E. (2008). Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells, 26(5), 1325–1336. https://doi.org/10.1634/stemcells.2007-1072.
Gu, S., Jin, L., Zhang, F., Sarnow, P., & Kay, M. A. (2009). Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nature Structural and Molecular Biology, 16(2), 144–150. https://doi.org/10.1038/nsmb.1552.
Hwang, H. W., & Mendell, J. T. (2006). MicroRNAs in cell proliferation, cell death, and tumorigenesis. British Journal of Cancer, 94(6), 776–780. https://doi.org/10.1038/sj.bjc.6603023.
Li, N., Long, B., Han, W., Yuan, S., & Wang, K. (2017). MicroRNAs: Important regulators of stem cells. Stem Cell Research and Therapy, 8(1), 1–7. https://doi.org/10.1186/s13287-017-0551-0.
O’Brien, J., Hayder, H., Zayed, Y., & Peng, C. (2018). Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology, 9, 402. https://doi.org/10.3389/fendo.2018.00402.
Zhao, X. L., Yang, B., Ma, L. N., & Dong, Y. H. (2016). MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells. Artificial Cells, Nanomedicine and Biotechnology, 44(7), 1665–1670. https://doi.org/10.3109/21691401.2015.1080168.
Shen, X., Pan, B., Zhou, H., Liu, L., Lv, T., Zhu, J., Huang, X., & Tian, J. (2017). Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. Journal of Biomedical Science, 24(1), 1–8. https://doi.org/10.1186/s12929-017-0337-9.
Dong, J., Zhang, Z., Huang, H., Mo, P., Cheng, C., Liu, J., Huang, W., Tian, C., Zhang, C., & Li, J. (2018). MiR-10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Research and Therapy, 9(1), 1–16. https://doi.org/10.1186/s13287-018-0895-0.
Chen, Y., Zhao, Y., Chen, W., Xie, L., Zhao, Z. A., Yang, J., Chen, Y., Lei, W., & Shen, Z. (2017). MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Research and Therapy, 8(1), 1–11. https://doi.org/10.1186/s13287-017-0722-z.
Zhang, Y., Lei, W., Yan, W., Li, X., Wang, X., Zhao, Z., Hui, J., Shen, Z., & Yang, J. (2016). MicroRNA-206 is involved in survival of hypoxia preconditioned mesenchymal stem cells through targeting Pim-1 kinase. Stem Cell Research and Therapy, 7(1), 1–10. https://doi.org/10.1186/s13287-016-0318-z.
Mohtavinejad, N., Nakhaee, A., Harati, H., Poodineh, J., & Afzali, M. (2015). SIRT1 gene is associated with cardiovascular disease in the Iranian population. Egyptian Journal of Medical Human Genetics, 16(2), 117–122. https://doi.org/10.1016/j.ejmhg.2014.11.005.
Zhang, F., Cui, J., Liu, X., Lv, B., Liu, X., Xie, Z., & Yu, B. (2015). Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Research and Therapy, 6(1), 1–13. https://doi.org/10.1186/s13287-015-0187-x.
Guo, X., Bai, Y., Zhang, L., Zhang, B., Zagidullin, N., Carvalho, K., Du, Z., & Cai, B. (2018). Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Research and Therapy, 9(1), 1–12. https://doi.org/10.1186/s13287-018-0773-9.
Mohanty, S., Bose, S., Jain, K. G., Bhargava, B., & Airan, B. (2013). TGFβ1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. International Journal of Cardiology, 163(1), 93–99. https://doi.org/10.1016/j.ijcard.2011.08.003.
Rouhi, L., Kajbafzadeh, A. M., Modaresi, M., Shariati, M., & Hamrahi, D. (2013). Autologous serum enhances cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells in the presence of transforming growth factor-beta1 (TGF-beta1). In Vitro Cellular & Developmental Biology. Animal, 49(4), 287–294. https://doi.org/10.1007/s11626-013-9597-1.
Kumar, D., & Sun, B. (2005). Transforming growth factor-β2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochemical and Biophysical Research Communications, 332(1), 135–141. https://doi.org/10.1016/J.BBRC.2005.04.098.
Ma, L.-L., Wang, N., Zhou, Z., Zhang, J.-Y., Luo, X.-G., Jiang, Y., & Zhang, T.-C. (2009). Transforming growth factor-β3 induced rat bone marrow-derived mesenchymal stem cells differentiation into smooth muscle cells by activating Myocardin. Journal of Biomedical Science and Engineering, 02(08), 651–655. https://doi.org/10.4236/jbise.2009.28095.
Lv, Y., Gao, C. W., Liu, B., Wang, H. Y., & Wang, H. P. (2017). BMP-2 combined with salvianolic acid B promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Kaohsiung Journal of Medical Sciences, 33(10), 477–485. https://doi.org/10.1016/j.kjms.2017.06.006.
Zhang, G. W., Gu, T. X., Guan, X. Y., Sun, X. J., Qi, X., Li, X. Y., Wang, X. B., Lv, F., Yu, L., Jiang, D. Q., & Tang, R. (2015). HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Proliferation, 48(6), 661–670. https://doi.org/10.1111/cpr.12219.
Hafez, P., Jose, S., Chowdhury, S. R., Ng, M. H., Ruszymah, B. H. I., & Mohd, R. A. R. (2016). Cardiomyogenic differentiation of human sternal bone marrow mesenchymal stem cells using a combination of basic fibroblast growth factor and hydrocortisone. Cell Biology International, 40(1), 55–64. https://doi.org/10.1002/cbin.10536.
Azhar, M., Schultz, J. E. J., Grupp, I., Dorn, G. W. II, Meneton, P., Molin, D. G. M., Gittenberger-de Groot, A. C., & Doetschman, T. (2003). Transforming growth factor beta in cardiovascular development and function. Cytokine & Growth Factor Reviews, 14(5), 391–407. https://doi.org/10.1016/s1359-6101(03)00044-3.
Tao-Sheng, L., Masanori, H., Hiroshi, I., Akira, F., Tomoaki, M., Masunori, M., & Kimikazu, H. (2005). Regeneration of infarcted myocardium by Intramyocardial Implantation of ex vivo transforming growth factor-β–preprogrammed bone marrow stem cells. Circulation, 111(19), 2438–2445. https://doi.org/10.1161/01.CIR.0000167553.49133.81.
Ahadian, S., Ostrovidov, S., Hosseini, V., Kaji, H., Ramalingam, M., Bae, H., & Khademhosseini, A. (2013). Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis, 9(2), 87–92. https://doi.org/10.4161/org.25121.
He, X., Li, L., Tang, M., Zeng, Y., Li, H., & Yu, X. (2019). Biomimetic electrical stimulation induces rat bone marrow mesenchymal stem cells to differentiate into cardiomyocyte-like cells via TGF-beta 1 in vitro. Progress in Biophysics and Molecular Biology, 148, 47–53. https://doi.org/10.1016/j.pbiomolbio.2017.09.023.
Salazar, V. S., Gamer, L. W., & Rosen, V. (2016). BMP signalling in skeletal development, disease and repair. Nature Reviews Endocrinology, 12(4), 203–221. https://doi.org/10.1038/nrendo.2016.12.
van Wijk, B., Moorman, A. F. M., & van den Hoff, M. J. B. (2007). Role of bone morphogenetic proteins in cardiac differentiation. Cardiovascular Research, 74(2), 244–255. https://doi.org/10.1016/j.cardiores.2006.11.022.
Ebelt, H., Hillebrand, I., Arlt, S., Zhang, Y., Kostin, S., Neuhaus, H., Müller-Werdan, U., Schwarz, E., Werdan, K., & Braun, T. (2013). Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock (Augusta, Ga.), 39(4), 353–360. https://doi.org/10.1097/SHK.0b013e318289728a.
Liu, C., Chen, L., Zeng, J., Cui, J., Ning, J., Wang, G., song, Belguise, K., Wang, X., sheng Qian, G., Lu, K., & Yi, B. (2015). Bone morphogenic protein-2 regulates the myogenic differentiation of PMVECs in CBDL rat serum-induced pulmonary microvascular remodeling. Experimental Cell Research, 336(1), 109–118. https://doi.org/10.1016/j.yexcr.2015.05.025.
Zhang, Z., Li, H., Ma, Z., Feng, J., Gao, P., Dong, H., & Zhang, Z. (2012). Efficient cardiomyogenic differentiation of bone marrow mesenchymal stromal cells by combination of Wnt11 and bone morphogenetic protein 2. Experimental Biology and Medicine, 237(7), 768–776. https://doi.org/10.1258/ebm.2012.011291.
Wang, Y., li, Zhang, G., Wang, H., Tan, Y. zhen, & Wang, X. (2018). Preinduction with bone morphogenetic protein-2 enhances cardiomyogenic differentiation of c-kit + mesenchymal stem cells and repair of infarcted myocardium. International Journal of Cardiology, 265, 173–180. https://doi.org/10.1016/j.ijcard.2018.01.134.
Hoxha, E., Lambers, E., Wasserstrom, J. A., Mackie, A., Ramirez, V., Abramova, T., Verma, S. K., Krishnamurthy, P., & Kishore, R. (2012). Elucidation of a novel pathway through Which HDAC1 controls cardiomyocyte differentiation through expression of SOX-17 and BMP2. PLoS One, 7(9). https://doi.org/10.1371/journal.pone.0045046.
Chan, S. S. K., Chen, J. H., Hwang, S. M., Wang, I. J., Li, H. J., Lee, R. T., & Hsieh, P. C. H. (2009). Salvianolic acid B-vitamin C synergy in cardiac differentiation from embryonic stem cells. Biochemical and Biophysical Research Communications, 387(4), 723–728. https://doi.org/10.1016/j.bbrc.2009.07.122.
Yu, J., Chen, R., Tan, Y., Wu, J., Qi, J., Zhang, M., & Gu, W. (2016). Salvianolic acid B alleviates heart failure by inactivating ERK1/2/GATA4 signaling pathway after pressure overload in mice. PLoS One, 11(11), 1–16. https://doi.org/10.1371/journal.pone.0166560.
Suleiman, M. S., Singh, R. J. R., & Stewart, C. E. H. (2007). Apoptosis and the cardiac action of insulin-like growth factor I. Pharmacology and Therapeutics, 114(3), 278–294. https://doi.org/10.1016/j.pharmthera.2007.03.001.
Gong, H., Wang, X., Wang, L., Liu, Y., Wang, J., Lv, Q., Pang, H. I., Zhang, Q., & Wang, Z. (2017). Inhibition of IGF-1 receptor kinase blocks the differentiation into cardiomyocyte-like cells of BMSCs induced by IGF-1. Molecular Medicine Reports, 16(1), 787–793. https://doi.org/10.3892/mmr.2017.6639.
Haider, H. K., Jiang, S., Idris, N. M., & Ashraf, M. (2008). IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circulation Research, 103(11), 1300–1308. https://doi.org/10.1161/CIRCRESAHA.108.186742.
Miyahara, Y., Nagaya, N., Kataoka, M., Yanagawa, B., Tanaka, K., Hao, H., Ishino, K., Ishida, H., Shimizu, T., Kangawa, K., Sano, S., Okano, T., Kitamura, S., & Mori, H. (2006). Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine, 12(4), 459–465. https://doi.org/10.1038/nm1391.
Savi, M., Bocchi, L., Fiumana, E., Karam, J. P., Frati, C., Bonafé, F., Cavalli, S., Morselli, P. G., Guarnieri, C., Caldarera, C. M., Muscari, C., Montero-Menei, C. N., Stilli, D., Quaini, F., & Musso, E. (2015). Enhanced engraftment and repairing ability of human adipose-derived stem cells, conveyed by pharmacologically active microcarriers continuously releasing HGF and IGF-1, in healing myocardial infarction in rats. Journal of Biomedical Materials Research - Part A, 103(9), 3012–3025. https://doi.org/10.1002/jbm.a.35442.
Gómez-Mauricio, G., Moscoso, I., Martín-Cancho, M. F., Crisóstomo, V., Prat-Vidal, C., Báez-Díaz, C., Sánchez-Margallo, F. M., & Bernad, A. (2016). Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Research and Therapy, 7(1), 1–18. https://doi.org/10.1186/s13287-016-0350-z.
Yao, J., Ke, J., Zhou, Z., Tan, G., Yin, Y., Liu, M., Chen, J., & Wu, W. (2019). Combination of HGF and IGF-1 promotes connexin 43 expression and improves ventricular arrhythmia after myocardial infarction through activating the MAPK/ERK and MAPK/p38 signaling pathways in a rat model. Cardiovascular Diagnosis and Therapy, 9(4), 346–354. https://doi.org/10.21037/cdt.2019.07.12.
Sugi, Y., Sasse, J., Barron, M., & Lough, J. (1995). Developmental expression of fibroblast growth factor receptor-1 (cek‐1; flg) during heart development. Developmental Dynamics, 202(2), 115–125. https://doi.org/10.1002/aja.1002020203.
Khezri, S., Valojerdi, M. R., Sepehri, H., & Baharvand, H. (2007). Effect of basic fibroblast growth factor on cardiomyocyte differentiation from mouse embryonic stem cells. Saudi Medical Journal, 28(2), 181–186.
Itoh, N., Ohta, H., Nakayama, Y., & Konishi, M. (2016). Roles of FGF signals in heart development, health, and disease. Frontiers in Cell and Developmental Biology, 4(OCT), 1–11. https://doi.org/10.3389/fcell.2016.00110.
Rosenblatt-Velin, N., Lepore, M. G., Cartoni, C., Beermann, F., & Pedrazzini, T. (2005). FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. Journal of Clinical Investigation, 115(7), 1724–1733. https://doi.org/10.1172/JCI23418.
Song, H., Kwon, K., Lim, S., Kang, S. M., Ko, Y. G., Xu, Z., Chung, J. H., Kim, B. S., Lee, H., Joung, B., Park, S., Choi, D., Jang, Y., Chung, N. S., Yoo, K. J., & Hwang, K. C. (2005). Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Molecules and Cells, 19(3), 402–407.
Beenken, A., & Mohammadi, M. (2009). The FGF family: Biology, pathophysiology and therapy. Nature Reviews Drug Discovery, 8(3), 235–253. https://doi.org/10.1038/nrd2792.
Schmidt, A., Ladage, D., Schinköthe, T., Klausmann, U., Ulrichs, C., Klinz, F.-J., Brixius, K., Arnhold, S., Desai, B., Mehlhorn, U., Schwinger, R. H. G., Staib, P., Addicks, K., & Bloch, W. (2006). Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells, 24(7), 1750–1758. https://doi.org/10.1634/stemcells.2005-0191.
Rodrigues, M., Griffith, L. G., & Wells, A. (2010). Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Research & Therapy, 1(4), 32. https://doi.org/10.1186/scrt32.
Wang, X., Zhen, L., Miao, H., Sun, Q., Yang, Y., Que, B., Lao, E. P. L., Wu, X., Ren, H., Shi, S., Lau, W. B., Ma, X., Ma, C., & Nie, S. (2015). Concomitant retrograde coronary venous infusion of basic fibroblast growth factor enhances engraftment and differentiation of bone marrow mesenchymal stem cells for cardiac repair after myocardial infarction. Theranostics, 5(9), 995–1006. https://doi.org/10.7150/thno.11607.
Giraud, G. D., Louey, S., Jonker, S., Schultz, J., & Thornburg, K. L. (2006). Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology, 147(8), 3643–3649. https://doi.org/10.1210/en.2006-0061.
Malandraki-Miller, S., Lopez, C. A., Al-Siddiqi, H., & Carr, C. A. (2018). Changing metabolism in differentiating cardiac progenitor cells—can stem cells become metabolically flexible cardiomyocytes? Frontiers in Cardiovascular Medicine, 5(September), 1–21. https://doi.org/10.3389/fcvm.2018.00119.
Fenaux, P., Mufti, G. J., Hellstrom-Lindberg, E., Santini, V., Gattermann, N., Germing, U., Sanz, G., List, A. F., Gore, S., Seymour, J. F., Dombret, H., Backstrom, J., Zimmerman, L., McKenzie, D., Beach, C. L., & Silverman, L. R. (2010). Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 28(4), 562–569. https://doi.org/10.1200/JCO.2009.23.8329.
Makino, S., Fukuda, K., Miyoshi, S., Konishi, F., Kodama, H., Pan, J., Sano, M., Takahashi, T., Hori, S., Abe, H., Hata, J., Umezawa, A., & Ogawa, S. (1999). Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of Clinical Investigation, 103(5), 697–705. https://doi.org/10.1172/JCI5298.
Antonitsis, P., Ioannidou-Papagiannaki, E., Kaidoglou, A., & Papakonstantinou, C. (2007). In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interactive Cardiovascular and Thoracic Surgery, 6(5), 593–597. https://doi.org/10.1510/icvts.2007.157875.
Govarthanan, K., Kumar, P., Ramasamy, D., & Kumar, P. (2019). Genomics DNA methylation microarray uncovers a permissive methylome for cardiomyocyte di ff erentiation in human mesenchymal stem cells. Genomics, June, 0–1. https://doi.org/10.1016/j.ygeno.2019.08.007.
Jasmin, Spray, D. C., De Carvalho, A. C. C., & Mendez-Otero, R. (2010). Chemical induction of cardiac differentiation in P19 embryonal carcinoma stem cells. Stem Cells and Development, 19(3), 403–411. https://doi.org/10.1089/scd.2009.0234.
Feng, C., Zhu, J., Zhao, L., Lu, T., Zhang, W., Liu, Z., & Tian, J. (2009). Suberoylanilide hydroxamic acid promotes cardiomyocyte differentiation of rat mesenchymal stem cells. Experimental Cell Research, 315(17), 3044–3051. https://doi.org/10.1016/j.yexcr.2009.05.005.
Lim, S. Y., Sivakumaran, P., Crombie, D. E., Dusting, G. J., Pébay, A., & Dilley, R. J. (2013). Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Translational Medicine, 2(9), 715–725. https://doi.org/10.5966/sctm.2012-0161.
Hatami, L., Valojerdi, M. R., & Mowla, S. J. (2007). Effects of oxytocin on cardiomyocyte differentiation from mouse embryonic stem cells. International Journal of Cardiology, 117(1), 80–89. https://doi.org/10.1016/j.ijcard.2006.04.054.
Dal-pra, S., Hodgkinson, C. P., Mirotsou, M., Kirste, I., & Dzau, V. J. (2016). Demethylation of H3K27 Is Essential for the Induction of Direct Cardiac Reprogramming by miR Combo. January. https://doi.org/10.1161/CIRCRESAHA.116.308741.
Gao, Q., Guo, M., Jiang, X., Hu, X., Wang, Y., & Fan, Y. (2014). A cocktail method for promoting cardiomyocyte differentiation from bone marrow-derived mesenchymal stem cells. Stem Cells International, 2014, 162024. https://doi.org/10.1155/2014/162024.
Wan Safwani, W. K. Z., Makpol, S., Sathapan, S., & Chua, K. (2012). 5-azacytidine is insufficient for cardiogenesis in human adipose-derived stem cells. Journal of Negative Results in BioMedicine, 11(1), 1–9. https://doi.org/10.1186/1477-5751-11-3.
Zhou, G. S., Zhang, X. L., Wu, J. P., Zhang, R. P., Xiang, L. X., Dai, L. C., & Shao, J. Z. (2009). 5-Azacytidine facilitates osteogenic gene expression and differentiation of mesenchymal stem cells by alteration in DNA methylation. Cytotechnology, 60(1–3), 11–22. https://doi.org/10.1007/s10616-009-9203-2.
Bhuvanalakshmi, G., Arfuso, F., Kumar, A. P., Dharmarajan, A., & Warrier, S. (2017). Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Research & Therapy, 8(1), 185. https://doi.org/10.1186/s13287-017-0638-7.
He, A., Shen, X., Ma, Q., Cao, J., von Gise, A., Zhou, P., Wang, G., Marquez, V. E., Orkin, S. H., & Pu, W. T. (2012). PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes & Development, 26(1), 37–42. https://doi.org/10.1101/gad.173930.111.
Dal-Pra, S., Hodgkinson, C. P., Mirotsou, M., Kirste, I., & Dzau, V. J. (2017). Demethylation of H3K27 is essential for the induction of direct cardiac reprogramming by miR combo. Circulation Research, 120(9), 1403–1413. https://doi.org/10.1161/CIRCRESAHA.116.308741.
Krämer, M., Dees, C., Huang, J., Schlottmann, I., Palumbo-Zerr, K., Zerr, P., Gelse, K., Beyer, C., Distler, A., Marquez, V. E., Distler, O., Schett, G., & Distler, J. H. W. (2013). Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Annals of the Rheumatic Diseases, 72(4), 614 LP–620. https://doi.org/10.1136/annrheumdis-2012-201615.
Lu, D. F., Wang, Y., Su, Z. Z., Zeng, Z. H., Xing, X. W., He, Z. Y., & Zhang, C. (2014). Knockdown of the HDAC1 promotes the directed differentiation of bone mesenchymal stem cells into cardiomyocytes. PLoS One, 9(3). https://doi.org/10.1371/journal.pone.0092179.
Li, L., Zhu, J., Tian, J., Liu, X., & Feng, C. (2010). A role for Gcn5 in cardiomyocyte differentiation of rat mesenchymal stem cells. Molecular and Cellular Biochemistry, 345(1–2), 309–316. https://doi.org/10.1007/s11010-010-0586-3.
Ostrovidov, S., Shi, X., Sadeghian, R. B., Salehi, S., Fujie, T., Bae, H., Ramalingam, M., & Khademhosseini, A. (2015). Stem Cell Differentiation Toward the Myogenic Lineage for Muscle Tissue Regeneration: A Focus on Muscular Dystrophy. Stem Cell Reviews and Reports, 11(6), 866–884. https://doi.org/10.1007/s12015-015-9618-4.
Gong, R., Jiang, Z., Zagidullin, N., Liu, T., & Cai, B. (2021). Regulation of cardiomyocyte fate plasticity: a key strategy for cardiac regeneration. Signal Transduction and Targeted Therapy, 6(1), 31. https://doi.org/10.1038/s41392-020-00413-2.
Lin, Y.-L., Chen, C.-P., Lo, C.-M., & Wang, H.-S. (2016). Stiffness-controlled three-dimensional collagen scaffolds for differentiation of human Wharton’s jelly mesenchymal stem cells into cardiac progenitor cells. Journal of Biomedical Materials Research. Part A, 104(9), 2234–2242. https://doi.org/10.1002/jbm.a.35762.
Wu, Y.-J., Chen, S. Y., Chang,S. J., & Kuo, S. M. (2013). Enhanced differentiation of rat MSCs intocardiomyocytes with 5-azacytidine/collagen I nano-molecules. Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 2013, 322–325. https://doi.org/10.1109/EMBC.2013.6609502.
Bagheri-Hosseinabadi, Z., Salehinejad, P., & Mesbah-Namin, S. A. (2017). Differentiation of human adipose-derived stem cells into cardiomyocyte-like cells in fibrin scaffold by a histone deacetylase inhibitor. BioMedical Engineering OnLine, 16(1), 134. https://doi.org/10.1186/s12938-017-0423-y.
Park, J., Park, S., Ryu, S., Bhang, S. H., Kim, J., Yoon, J.-K., Park, Y. H., Cho, S.-P., Lee, S., Hong, B. H., & Kim, B.-S. (2014). Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Advanced Healthcare Materials, 3(2), 176–181. https://doi.org/10.1002/adhm.201300177.
Lee, T.-J., Park, S., Bhang, S. H., Yoon, J.-K., Jo, I., Jeong, G.-J., Hong, B. H., & Kim, B.-S. (2014). Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochemical and Biophysical Research Communications, 452(1), 174–180. https://doi.org/10.1016/j.bbrc.2014.08.062.
Kai, D., Prabhakaran, M. P., Jin, G., Tian, L., & Ramakrishna, S. (2017). Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine, 11(4), 1002–1010. https://doi.org/10.1002/term.1999.
Crowder, S. W., Liang, Y., Rath, R., Park, A. M., Maltais, S., Pintauro, P. N., Hofmeister, W., Lim, C. C., Wang, X., & Sung, H.-J. (2013). Poly(ε-caprolactone)-carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine: the official journal of the American Academy of Nanomedicine, 8(11), 1763–1776. https://doi.org/10.2217/nnm.12.204.
Nart, J., de Tapia, B., Pujol, À, Pascual, A., & Valles, C. (2018). Vancomycin and tobramycin impregnated mineralized allograft for the surgical regenerative treatment of peri-implantitis: A 1-year follow-up case series. Clinical Oral Investigations, 22(6), 2199–2207. https://doi.org/10.1007/s00784-017-2310-0.
Rashedi, I., Talele, N., Wang, X. H., Hinz, B., Radisic, M., & Keating, A. (2017). Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS ONE, 12(10), 1–23. https://doi.org/10.1371/journal.pone.0187348.
Kim, H. N., Jiao, A., Hwang, N. S., Kim, M. S., Kang, D. H., Kim, D. H., & Suh, K. Y. (2013). Nanotopography-guided tissue engineering and regenerative medicine. Advanced Drug Delivery Reviews, 65(4), 536–558. https://doi.org/10.1016/j.addr.2012.07.014.
Lee, W. C., Lim, C. H. Y. X., Shi, H., Tang, L. A. L., Wang, Y., Lim, C. T., & Loh, K. P. (2011). Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano, 5(9), 7334–7341. https://doi.org/10.1021/nn202190c.
Kim, J., Kim, Y. R., Kim, Y., Lim, K. T., Seonwoo, H., Park, S., Cho, S. P., Hong, B. H., Choung, P. H., Chung, T. D., Choung, Y. H., & Chung, J. H. (2013). Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. Journal of Materials Chemistry B, 1(7), 933–938. https://doi.org/10.1039/c2tb00274d.
Kim, J., Choi, K. S., Kim, Y., Lim, K. T., Seonwoo, H., Park, Y., Kim, D. H., Choung, P. H., Cho, C. S., Kim, S. Y., Choung, Y. H., & Chung, J. H. (2013). Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. Journal of Biomedical Materials Research - Part A, 101(12), 3520–3530. https://doi.org/10.1002/jbm.a.34659.
Ahmed, M., & ffrench-Constant, C. (2016). Extracellular matrix regulation of stem cell behavior. Current Stem Cell Reports, 2(3), 197–206. https://doi.org/10.1007/s40778-016-0056-2.
Sreejit, P., & Verma, R. S. (2013). Natural ECM as biomaterial for scaffold based cardiac regeneration using adult bone marrow derived stem cells. Stem Cell Reviews and Reports, 9(2), 158–171. https://doi.org/10.1007/s12015-013-9427-6.
Sato, H., Takahashi, M., Ise, H., Yamada, A., Hirose, S. I., Tagawa, Y. I., Morimoto, H., Izawa, A., & Ikeda, U. (2006). Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochemical and Biophysical Research Communications, 342(1), 107–112. https://doi.org/10.1016/j.bbrc.2006.01.116.
Wang, Y., Wang, H., Liu, D., Song, S., Wang, X., & Zhang, H. (2013). Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials, 34(31), 7715–7724. https://doi.org/10.1016/j.biomaterials.2013.06.045.
Wang, L., Wu, Y., Hu, T., Guo, B., & Ma, P. X. (2017). Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomaterialia, 59, 68–81. https://doi.org/10.1016/j.actbio.2017.06.036.
Ahadian, S., Ramalingam, M., & Khademhosseini, A. (2013). The emerging applications of graphene oxide and graphene in tissue engineering. In Biomimetics (pp. 279–299). https://doi.org/10.1002/9781118810408.ch12.
Rashedi, I., Talele, N., Wang, X.-H., Hinz, B., Radisic, M., & Keating, A. (2017). Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PloS One, 12(10), e0187348. https://doi.org/10.1371/journal.pone.0187348.
Ingber, D. E. (2002). Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circulation Research, 91(10), 877–887. https://doi.org/10.1161/01.RES.0000039537.73816.E5.
Park, S. J., Kim, K. H., Jeon, W. Y., Seo, J., Han, J. M., Kim, J. S., Chung, H. M., Lee, J. H., Moon, S. H., & Kim, H. H. (2017). Enzyme catalyzed electrostimulation of human embryonic stem cell-derived cardiomyocytes influence contractility and synchronization. Biochemical Engineering Journal, 123, 95–109. https://doi.org/10.1016/j.bej.2017.03.013.
Rungarunlert, S., Klincumhom, N., Bock, I., Nemes, C., Techakumphu, M., Pirity, M. K., & Dinnyes, A. (2011). Enhanced cardiac differentiation of mouse embryonic stem cells by use of the slow-turning, lateral vessel (STLV) bioreactor. Biotechnology Letters, 33(8), 1565–1573. https://doi.org/10.1007/s10529-011-0614-8.
Yan, Z., Yang, G., Cui, L., He, X., Kuang, W., Wu, W., Liu, X., & Li, L. (2013). [Effects of electrical stimulation on the differentiation of mesenchymal stem cells into cardiomyocyte-like cells]. Sheng wu yi xue gong cheng xue za zhi = Journal of biomedical engineering = Shengwu yixue gongchengxue zazhi, 30(3), 556–561.
Kang, P.-L., Lin, Y.-H., Chen, S.-Y., Chu, J.-H., & Chang, S. J. (n.d.). The study of cardiac differentiation of mesenchymal stem cells by electrostimulation on the myocardial repair. Frontiers in Bioengineering and Biotechnology, 609. https://doi.org/10.3389/conf.FBIOE.2016.01.00609.
Sridhar, S., Venugopal, J. R., Sridhar, R., & Ramakrishna, S. (2015). Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers. Colloids and Surfaces B: Biointerfaces, 134, 346–354. https://doi.org/10.1016/j.colsurfb.2015.07.019.
Song, H., Hwang, J., Chang, W., Song, B.-W., Cha, M.-J., Kim, I.-K., Lim, S., Choi, E. J., Ham, O., Lee, C., Park, J.-H., Lee, S.-Y., Choi, E., Lee, C., Lee, M., Lee, M.-H., Kim, S.-H., Jang, Y., & Hwang, K.-C. (2011). Cardiomyocytes from phorbol myristate acetate-activated mesenchymal stem cells restore electromechanical function in infarcted rat hearts. Proceedings of the National Academy of Sciences of the United States of America, 108, 296–301. https://doi.org/10.1073/pnas.1015873107.
Engler, A. J., Griffin, M. A., Sen, S., Bönnemann, C. G., Sweeney, H. L., & Discher, D. E. (2004). Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. The Journal of Cell Biology, 166(6), 877–887. https://doi.org/10.1083/jcb.200405004.
Zimmermann, W. H., Schneiderbanger, K., Schubert, P., Didié, M., Münzel, F., Heubach, J. F., Kostin, S., Neuhuber, W. L., Eschenhagen, T. (2002). Tissue engineering of a differentiated cardiac muscle construct. Circulation Research, 90(2), 223–230. https://doi.org/10.1161/hh0202.103644.
Guan, J., Wang, F., Li, Z., Chen, J., Guo, X., Liao, J., & Moldovan, N. I. (2011). The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials, 32(24), 5568–5580. https://doi.org/10.1016/j.biomaterials.2011.04.038.
Tsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells, 9(4). https://doi.org/10.3390/cells9040991.
Bhartiya, D., Flora, Y., Sharma, D., & Mohammad, S. A. (2021). Two stem cell populations including VSELs and CSCs detected in the pericardium of adult mouse heart. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-021-10119-9.
Blaschuk, O. W., & Rowlands, T. M. (2000). Cadherins as modulators of angiogenesis and the structural integrity of blood vessels. Cancer and Metastasis Reviews, 19(1), 1–5. https://doi.org/10.1023/A:1026522216059.
Kishore, R., Qin, G., Luedemann, C., Bord, E., Hanley, A., Silver, M., Gavin, M., Goukassain, D., & Losordo, D. W. (2005). The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-α–induced transcriptional repression of cyclin A. The Journal of Clinical Investigation, 115(7), 1785–1796. https://doi.org/10.1172/JCI22849.
Wu, E., Palmer, N., Tian, Z., Moseman, A. P., Galdzicki, M., Wang, X., Berger, B., Zhang, H., & Kohane, I. S. (2008). Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PLoS One, 3(11), e3794–e3794. https://doi.org/10.1371/journal.pone.0003794.
Shibuya, M. (2011). Vascular Endothelial Growth Factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes & Cancer, 2(12), 1097–1105. https://doi.org/10.1177/1947601911423031.
Liu, L.-Z., Li, C., Chen, Q., Jing, Y., Carpenter, R., Jiang, Y., Kung, H.-F., Lai, L., & Jiang, B.-H. (2011). MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One, 6(4), e19139. https://doi.org/10.1371/journal.pone.0019139.
Feng, Y., Huang, W., Wani, M., Yu, X., & Ashraf, M. (2014). Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One, 9(2), e88685. https://doi.org/10.1371/journal.pone.0088685.
Qian, L., Van Laake, L. W., Huang, Y., Liu, S., Wendland, M. F., & Srivastava, D. (2011). miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. Journal of Experimental Medicine, 208(3), 549–560. https://doi.org/10.1084/jem.20101547.
Yu, B., Gong, M., Wang, Y., Millard, R. W., Pasha, Z., Yang, Y., Ashraf, M., & Xu, M. (2013). Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One, 8(8), e73304. https://doi.org/10.1371/journal.pone.0073304.
Liu, J.-S., Du, J., Cheng, X., Zhang, X.-Z., Li, Y., & Chen, X.-L. (2019). Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. Journal of the Chinese Medical Association, 82(12). https://journals.lww.com/jcma/Fulltext/2019/12000/Exosomal_miR_451_from_human_umbilical_cord.4.aspx.
Hong, Y., Cao, H., Wang, Q., Ye, J., Sui, L., Feng, J., Cai, X., Song, H., Zhang, X., & Chen, X. (2016). MiR-22 may suppress fibrogenesis by targeting TGFβR I in cardiac fibroblasts. Cellular Physiology and Biochemistry, 40(6), 1345–1353. https://doi.org/10.1159/000453187.
Maegdefessel, L., Spin, J. M., Raaz, U., Eken, S. M., Toh, R., Azuma, J., Adam, M., Nagakami, F., Heymann, H. M., Chernugobova, E., Jin, H., Roy, J., Hultgren, R., Caidahl, K., Schrepfer, S., Hamsten, A., Eriksson, P., McConnell, M. V., Dalman, R. L., & Tsao, P. S. (2014). miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nature Communications, 5(1), 5214. https://doi.org/10.1038/ncomms6214.
Zhao, J., Li, X., Hu, J., Chen, F., Qiao, S., Sun, X., Gao, L., Xie, J., & Xu, B. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research, 115(7), 1205–1216. https://doi.org/10.1093/cvr/cvz040.
Zhang, X., Yuan, X., Shi, H., Wu, L., Qian, H., & Xu, W. (2015). Exosomes in cancer: small particle, big player. Journal of Hematology & Oncology, 8(1), 83. https://doi.org/10.1186/s13045-015-0181-x.
Li, S., Li, Y., Chen, B., Zhao, J., Yu, S., Tang, Y., Zheng, Q., Li, Y., Wang, P., He, X., & Huang, S. (2018). exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Research, 46(D1), D106–D112. https://doi.org/10.1093/nar/gkx891.
Sebastião, M. J., Serra, M., Pereira, R., Palacios, I., Gomes-Alves, P., & Alves, P. M. (2019). Human cardiac progenitor cell activation and regeneration mechanisms: exploring a novel myocardial ischemia/reperfusion in vitro model. Stem Cell Research & Therapy, 10(1), 77. https://doi.org/10.1186/s13287-019-1174-4.
Singla, D. K., Lyons, G. E., & Kamp, T. J. (2007). Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. American Journal of Physiology-Heart and Circulatory Physiology, 293(2), H1308–H1314. https://doi.org/10.1152/ajpheart.01277.2006.
Marta, A., Guangming, C.,Sylwia, B.-W., Lin, Z., Sylwia, K.-K., Anweshan, S., Elzbieta, K., Yu-Ting, X.,Bozena, S.-R., Xing, C., Urszula, J., Magdy, G., Malgorzata, S., Arash, D.,Slawomir, L., J., V. R., Michal, S., L., N. K., Ou-Li, W., … K., Z.-S. E.(2018). Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circulation Research, 122(2), 296–309. https://doi.org/10.1161/CIRCRESAHA.117.311769.
Haider, K. H., & Aramini, B. (2020). Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy. Stem Cell Research & Therapy, 11(1), 23. https://doi.org/10.1186/s13287-019-1548-7.
Zhao, Y., Sun, X., Cao, W., Ma, J., Sun, L., Qian, H., Zhu, W., & Xu, W. (2015). Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells International, 2015, 761643. https://doi.org/10.1155/2015/761643.
Yin, K., Wang, S., & Zhao, R. C. (2019). Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Research, 7(1), 8. https://doi.org/10.1186/s40364-019-0159-x.
Yao, Y., Huang, J., Geng, Y., Qian, H., Wang, F., Liu, X., Shang, M., Nie, S., Liu, N., Du, X., Dong, J., & Ma, C. (2015). Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One, 10(6), e0129164–e0129164. https://doi.org/10.1371/journal.pone.0129164.
Bara, J. J., McCarthy, H. E., Humphrey, E., Johnson, W. E. B., & Roberts, S. (2013). Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: implications for bone and cartilage tissue engineering. Tissue Engineering Part A, 20(1–2), 147–159. https://doi.org/10.1089/ten.tea.2013.0196.
Zhu, L.-P., Tian, T., Wang, J.-Y., He, J.-N., Chen, T., Pan, M., Xu, L., Zhang, H.-X., Qiu, X.-T., Li, C.-C., Wang, K.-K., Shen, H., Zhang, G.-G., & Bai, Y.-P. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8(22), 6163–6177. https://doi.org/10.7150/thno.28021.
Bartosh, T. J., Ylöstalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., Lee, R. H., Choi, H., & Prockop, D. J. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences, 107(31), 13724 LP – 13729. https://doi.org/10.1073/pnas.1008117107.
Frith, J. E., Thomson, B., & Genever, P. G. (2009). Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Engineering Part C: Methods, 16(4), 735–749. https://doi.org/10.1089/ten.tec.2009.0432.
Redondo-Castro, E., Cunningham, C. J., Miller, J., Brown, H., Allan, S. M., & Pinteaux, E. (2018). Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Research & Therapy, 9(1), 11. https://doi.org/10.1186/s13287-017-0753-5.
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., Shang, X., Zhang, Z. G., & Chopp, M. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 30(7), 1556–1564. https://doi.org/10.1002/stem.1129.
Shao, L., Zhang, Y., Lan, B., Wang, J., Zhang, Z., Zhang, L., Xiao, P., Meng, Q., Geng, Y., Yu, X., & Li, Y. (2017). MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. BioMed Research International, 2017, 4150705. https://doi.org/10.1155/2017/4150705.
Tang, J., Jin, L., Liu, Y., Li, L., Ma, Y., Lu, L., Ma, J., Ding, P., Yang, X., Liu, J., & Yang, J. (2020). Exosomes derived from mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through inhibiting pyroptosis. Drug Design, Development and Therapy, 14, 3765–3775. https://doi.org/10.2147/DDDT.S239546.
Yang, Y., Li, Y., Chen, X., Cheng, X., Liao, Y., & Yu, X. (2016). Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. Journal of Molecular Medicine, 94(6), 711–724. https://doi.org/10.1007/s00109-016-1387-2.
Funding
This study was supported by VIT-SEED grant.
Author information
Authors and Affiliations
Contributions
Murugan Ramalingam conceived and planned the review design. Saravanan Ramesh contributed for literature survey and major manuscript writing. Kavitha Govarthanan, Serge Ostrovidov and Gulden Camci-Unal have played a key role in revising the manuscript. Haiguang Zhang, Qingxi Hu, Rama S Verma and Murugan Ramalingam have contributed for editing the manuscript at all the stages. All authors discussed and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
All authors declared their consent to publication.
Conflict of Interest
The authors declare no competing Interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliation of the authors, Kavitha Govarthanan and Rama S Verma, is incorrect.
Rights and permissions
About this article
Cite this article
Ramesh, S., Govarthanan, K., Ostrovidov, S. et al. Cardiac Differentiation of Mesenchymal Stem Cells: Impact of Biological and Chemical Inducers. Stem Cell Rev and Rep 17, 1343–1361 (2021). https://doi.org/10.1007/s12015-021-10165-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10165-3