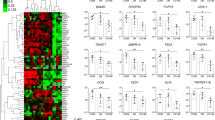
Abstract
Mesenchymal stem/ stromal cell (MSC) exhaustion has been suggested to be a hallmark of aging. Osteoarthritis has a complex etiology that comprises several factors. Dysplasia has been shown to be an individual risk factor for osteoarthritis. Subchondral bone changes are often the first detectable alterations in osteoarthritis. In this study, we aimed to determine whether skeletal MSCs are differentially affected in patients with primary versus dysplastic osteoarthritis. Patients undergoing hip arthroplasty due to primary osteoarthritis (n = 11) and osteoarthritis with hip dysplasia (n = 10) were included in the study. Femoral head subchondral bone was used for isolation of MSCs. The cells were compared using detailed ex-vivo and in-vitro analyses, which included immunophenotyping, colony-forming-unit fibroblast assay, growth kinetics, senescence, multilineage potential, immunophenotyping, and MSC marker-gene expression profiling. Isolated cells from primary osteoarthritis patients showed decreased viability in comparison with those from dysplasia patients, with similar mesenchymal fractions (i.e., CD45/ CD19/ CD14/ CD34-negative cells). In-vitro expanded MSCs from primary osteoarthritis patients showed reduced osteogenic and chondrogenic potential in comparison with dysplasia patients. There were no differences in clonogenicity, growth kinetics, senescence, adipogenic potential, and immunophenotype between these groups. Gene expression profiling showed well-known marker of bone marrow MSCs, the leptin receptor, to be significantly lower for primary osteoarthritis patients. Our study shows that the pathology of primary osteoarthritis is accompanied by bone MSC exhaustion, while biomechanical dysfunction associated with hip dysplasia can induce secondary osteoarthritis without this MSC impairment. Our study suggests that subchondral bone MSC exhaustion is implicated in the pathology of primary osteoarthritis.






Similar content being viewed by others
References
Campbell, T. M., Churchman, S. M., Gomez, A., McGonagle, D., Conaghan, P. G., Ponchel, F., & Jones, E. (2016). Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis & Rheumatology, 68, 1648–1659. https://doi.org/10.1002/art.39622.
De Bari, C., & Dell ‘Accio, F., Karystinou, A., et al. (2008). A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis and Rheumatism. https://doi.org/10.1002/art.23143.
De Bari, C., & Dell ‘Accio, F., Tylzanowski, P., Luyten, F. P. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism. https://doi.org/10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P.
Xu, L., Liu, Y., Sun, Y., Wang, B., Xiong, Y., Lin, W., & Li, G. (2017). Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Research & Therapy, 8, 1–11. https://doi.org/10.1186/s13287-017-0716-x.
Jayasuriya, C. T., Hu, N., Li, J., Lemme, N., Terek, R., Ehrlich, M. G., & Chen, Q. (2018). Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Scientific Reports, 8, 1–14. https://doi.org/10.1038/s41598-018-25395-8.
Chan, C. K. F., Seo, E. Y., Chen, J. Y., et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell. https://doi.org/10.1016/j.cell.2014.12.002.
Čamernik, K., Barlič, A., Drobnič, M., Marc, J., Jeras, M., & Zupan, J. (2018). Mesenchymal stem cells in the musculoskeletal system: From animal models to human tissue regeneration? Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-018-9800-6.
Worthley, D. L., Churchill, M., & Compton, et al. (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. https://doi.org/10.1016/j.cell.2014.11.042.
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G., & Morrison, S. J. (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.06.008.
Chan, C. K. F., Gulati, G. S., Sinha, R., et al. (2018). Identification of the human skeletal stem cell. Cell. https://doi.org/10.1016/j.cell.2018.07.029.
Murphy, J. M., Dixon, K., Beck, S., Fabian, D., Feldman, A., & Barry, F. (2002). Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis and Rheumatism. https://doi.org/10.1002/art.10118.
Lories, R. J., & Luyten, F. P. (2011). The bone–cartilage unit in osteoarthritis. Nature Reviews Rheumatology. https://doi.org/10.1038/nrrheum.2010.197.
Goldring, S. R., & Goldring, M. B. (2016). Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nature Reviews Rheumatology. https://doi.org/10.1038/nrrheum.2016.148.
Jacobsen, S., & Sonne-Holm, S. (2005). Hip dysplasia: A significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology. https://doi.org/10.1093/rheumatology/keh436.
Jacobsen, S. (2006). Adult hip dysplasia and osteoarthritis. Studies in radiology and clinical epidemiology. Acta Orthopaedica. Supplementum, 77(324), 1–37.
McGonagle, D., Baboolal, T. G., & Jones, E. (2017). Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nature Reviews Rheumatology, 13, 719–730. https://doi.org/10.1038/nrrheum.2017.182.
Sakaguchi, Y., Sekiya, I., Yagishita, K., Ichinose, S., Shinomiya, K., & Muneta, T. (2009). Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Stem Cells. https://doi.org/10.1182/blood-2003-12-4452.
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675.
Gregory, C. A., Grady Gunn, W., Peister, A., & Prockop, D. J. (2004). An alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Analytical Biochemistry. https://doi.org/10.1016/j.ab.2004.02.002.
Grogan, S. P., Barbero, A., Winkelmann, Rieser, F., Fitzsimmons, J. S., O’Driscoll, S., Martin, I., Mainil-Varlet, P. (2005). Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Engineering 2006; 12(8):2141–9.
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., & Wittwer, C. T. (2009). The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. https://doi.org/10.1373/clinchem.2008.112797.
Andersen, C. L., Jensen, J. L., & Ørntoft, T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-04-0496.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. https://doi.org/10.1080/14653240600855905.
Partridge, L., Deelen, J., & Slagboom, P. E. (2018). Facing up to the global challenges of ageing. Nature. https://doi.org/10.1038/s41586-018-0457-8.
Tarte, K., Gaillard, J., Lataillade, J.-J., et al. (2009). Clinical-grade production of human mesenchymal stromal cells: Occurrence of aneuploidy without transformation. Blood. https://doi.org/10.1182/blood-2009-05-219907.
Zhou, S., Greenberger, J. S., Epperly, M. W., Goff, J. P., Adler, C., Leboff, M. S., & Glowacki, J. (2008). Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. https://doi.org/10.1111/j.1474-9726.2008.00377.x.
Mendicino, M., Bailey, A. M., Wonnacott, K., Puri, R. K., & Bauer, S. R. (2014). MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.01.013.
Kohno, Y., Mizuno, M., Ozeki, N., Katano, H., Komori, K., Fujii, S., Otabe, K., Horie, M., Koga, H., Tsuji, K., Matsumoto, M., Kaneko, H., Takazawa, Y., Muneta, T., & Sekiya, I. (2017). Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Research and Therapy, 8, 1–11. https://doi.org/10.1186/s13287-017-0572-8.
Crisan, M., Yap, S., Casteilla, L., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. https://doi.org/10.1016/j.stem.2008.07.003.
Futami, I., Ishijima, M., Kaneko, H., et al. (2012). Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. https://doi.org/10.1371/journal.pone.0045517.
Cottle, B. J., Lewis, F. C., Shone, V., & Ellison-Hughes, G. M. (2017). Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Research and Therapy, 8, 1–16. https://doi.org/10.1186/s13287-017-0612-4.
Arrighi, N., Moratal, C., Clément, N., et al. (2015). Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death & Disease. https://doi.org/10.1038/cddis.2015.79.
Zainabadi, K. (2018). The variable role of SIRT1 in the maintenance and differentiation of mesenchymal stem cells. Regenerative Medicine. https://doi.org/10.2217/rme-2017-0128.
Vrtačnik, P., Zupan, J., Mlakar, V., Kranjc, T., Marc, J., Kern, B., & Ostanek, B. (2018). Epigenetic enzymes influenced by oxidative stress and hypoxia mimetic in osteoblasts are differentially expressed in patients with osteoporosis and osteoarthritis. Scientific Reports, 8, 1–12. https://doi.org/10.1038/s41598-018-34255-4.
Acknowledgements
The authors thank Gorana Furlanič and Helena Poniž for their valuable assistance at Valdoltra Orthopaedic Hospital, Laura Poženel, Tina Levstek, Sandra Mramor, Iva Blaževič, Kyriaki Hadjianastasi, Lina Andersson, Matjaž Jeras and Majda Sirnik for their much appreciated technical and general help. The authors acknowledge Chris Berrie for scientific English editing of the manuscript.
Funding
This study was funded by the Slovenian Research Agency, P3–298 Research Programme and J3–7245 research project and partially funded by the ARTE Project EU INTERREG ITALIA SLOVENIA 2014 2020 and J3–1749 research project funded by the Slovenian Research Agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Klemen Čamernik, Anže Mihelič, Rene Mihalič, Rihard Trebše, Darja Marolt Presen and Janja Zupan. The first draft of the manuscript was written by Klemen Čamernik and Janja Zupan, and all of the authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
R. Trebše declares payment for development of educational presentations for Zimmer, Medacta, De Puy, Link and Hareus, and R. Mihalič declares consultancy for Medacta International. The rest of the authors declare that they have no conflicts of interest regarding this study.
Ethics Approval and Consent to Participate
Approval for this study was obtained from the National Medical Ethics Committee of the Republic of Slovenia (reference numbers: 0120–523/2016–2, KME 45/10/16). Written informed consent was obtained from all of the donors included in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Selection of the best reference gene for qPCR for adipogenesis and osteogenesis. NormFinder [22] was used to select the most stable reference gene between the treated cells and the controls for adipogenesis and osteogenesis, in a subset of 32 samples. The best combination of the three genes was GAPDH, RPL13A and RPLP0. Shown is the expression of the osteogenic genes (COL1A1, OC, parathyroid hormone 1 receptor [PTHR1], RUNX2) in another subset of 10 pairs of MSC samples treated with osteogenic media. There was no difference in the normalization of these genes to all three reference genes (GAPDH, RPL13A and RPLP0) and to GAPDH only (two-way ANOVA with Bonferroni correction: p = 0.3003 for COL1A1; p > 0.9999 for OC, PTHR1 and RUNX2). (PNG 242 kb)
ESM 2
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Čamernik, K., Mihelič, A., Mihalič, R. et al. Increased Exhaustion of the Subchondral Bone-Derived Mesenchymal Stem/ Stromal Cells in Primary Versus Dysplastic Osteoarthritis. Stem Cell Rev and Rep 16, 742–754 (2020). https://doi.org/10.1007/s12015-020-09964-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09964-x