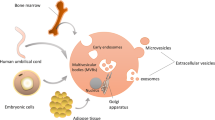
Abstract
Adult stem cells have beneficial effects when exposed to damaged tissue due, at least in part, to their paracrine activity, which includes soluble factors and extracellular vesicles (EVs). Given the multiplicity of signals carried by these vesicles through the horizontal transfer of functional molecules, human mesenchymal stem cell (hMSCs) and CD133+ cell-derived EVs have been tested in various disease models and shown to recover damaged tissues. In this study, we profiled the protein content of EVs derived from expanded human CD133+ cells and bone marrow-derived hMSCs with the intention of better understanding the functions performed by these vesicles/cells and delineating the most appropriate use of each EV in future therapeutic procedures. Using LC-MS/MS analysis, we identified 623 proteins for expanded CD133+-EVs and 797 proteins for hMSCs-EVs. Although the EVs from both origins were qualitatively similar, when protein abundance was considered, hMSCs-EVs and CD133+-EVs were different. Gene Ontology (GO) enrichment analysis in CD133+-EVs revealed proteins involved in a variety of angiogenesis-related functions as well proteins related to the cytoskeleton and highly implicated in cell motility and cellular activation. In contrast, when overrepresented proteins in hMSCs-EVs were analyzed, a GO cluster of immune response-related genes involved with immune response-regulating factors acting on phagocytosis and innate immunity was identified. Together our data demonstrate that from the point of view of protein content, expanded CD133+-EVs and hMSCs-EVs are in part similar but also sufficiently different to reflect the main beneficial paracrine effects widely reported in pre-clinical studies using expanded CD133+ cells and/or hBM-MSCs.




Similar content being viewed by others
References
Gnecchi, M., Zhang, Z., Ni, A., & Dzau, V. J. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research, 103(11), 1204–1219.
Lázaro-Ibáñez, E., Sanz-Garcia, A., Visakorpi, T., Escobedo-Lucea, C., Siljander, P., & Ayuso-Sacido, A. (2014). Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate, 74(14), 1379–1390.
Urbanelli, L., Magini, A., Buratta, S., Brozzi, A., Sagini, K., Polchi, A., Tancini, B., & Emiliani, C. (2013). Signaling pathways in exosomes biogenesis, secretion and fate. Genes, 4(2), 152–170.
Gatti, S., Bruno, S., Deregibus, M. C., Sordi, A., Cantaluppi, V., Tetta, C., & Camussi, G. (2011). Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion-induced acute and chronic kidney injury. Nephrology, Dialysis, Transplantation, 26(5), 1474–1483.
Cantaluppi, V., Gatti, S., Medica, D., Figliolini, F., Bruno, S., Deregibus, M. C., Sordi, A., Biancone, L., Tetta, C., & Camussi, G. (2012). Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney International, 82(4), 412–427.
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., & Wang, X. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 30(7), 1556–1564.
Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., & Cui, Y. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells, 31(12), 2737–2746.
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S., Choo, A., & Chen, T. S. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222.
Arslan, F., Lai, R. C., Smeets, M. B., Akeroyd, L., Choo, A., & Aguor, E. N. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10(3), 301–312.
Bian, S., Zhang, L., Duan, L., Wang, X., Min, Y., & Yu, H. (2014). Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of Molecular Medicine, 92(4), 387–397.
Kim, H. S., Choi, D. Y., Yun, S. J., Choi, S. M., Kang, J. W., Jung, J. W., Hwang, D., Kim, K. P., & Kim, D. W. (2012). Proteomic analysis of microvesicles derived from human mesenchymal stem cells. Journal of Proteome Research, 11(2), 839–849.
Anderson, J. D., Johansson, H. J., Graham, C. S., Vesterlund, M., Pham, M. T., Bramlett, C. S., Montgomery, E. N., et al. (2016). Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells, 34(3), 601–613.
Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., & Isner, J. M. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science, 275(5302), 964–966.
Asahara, T., Takahashi, T., Masuda, H., Kalka, C., Chen, D., Iwaguro, H., Inai, Y., Silver, M., & Isner, J. M. (1999). VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO Journal, 18(14), 3964–3972.
Chen, X., Armstrong, M. A., & Li, G. (2006). Mesenchymal stem cells in immunoregulation. Immunology and Cell Biology, 84, 413–421.
Rebelatto, C. K., Aguiar, A. M., Moretão, M. P., Senegaglia, A. C., Hansen, P., Barchiki, F., Oliveira, J., et al. (2008). Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Experimental Biology and Medicine, 233(7), 901–913.
Senegaglia, A. C., Barboza, L. A., Dallagiovanna, B., Aita, C. A., Hansen, P., Rebelatto, C. L., Aguiar, A. M., et al. (2010). Are purified or expanded cord blood-derived CD133+ cells better at improving cardiac function? Experimental Biology and Medicine, 235(1), 119–129.
FlowJo software, http://www.flowjo.com/, 2015. Accessed 14 April 2015.
Cox, J., & Mann, M. (2008). MaxQuant enables high peptide identification rates, individualized p.P.B.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology, 26, 1367–1372.
Cox, J., Neuhauser, N., Michalski, A., Scheltema, R. A., Olsen, J. V., & Mann, M. (2011). Andromeda: a peptide search engine integrated into the MaxQuant environment. Journal of Proteome Research, 10(4), 1794–1805.
Luber, C. A., Cox, J., Lauterbach, H., Fancke, B., Selbach, M., & Tschopp, J. (2010). Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity, 32(2), 279–289.
Pathan, M., Keerthikumar, S., Ang, C. S., Gangoda, L., Quek, C. M. J., Williamson, N. J., Mouradov, D., et al. (2015). FunRich: a standalone tool for functional enrichment analysis. Proteomics, 15(15), 2597–2601.
G:profiler software, http://biit.cs.ut.ee/gprofiler/, 2016. Accessed 10 May 2016.
Revigo software, http://revigo.irb.hr/, 2016. Accessed 03 July 2016.
Tyanova, S., Temu, T., Sinitcyn, P., Carlson, A., Hein, M., Geiger, T., Mann, M., et al. (2016). The Perseus computational platform for comprehensive analysis of (prote)omics data. Nature Methods, 13(9), 731–740.
Reus, T. L., Robert, A. W., Da Costa, M. B., Aguiar, A. M., & Stimamiglio, M. A. (2016). Secretome from resident cardiac stromal cells stimulates proliferation, cardiomyogenesis and angiogenesis of progenitor cells. International Journal of Cardiology, 15(221), 396–403.
Rao, A. V., & Shaha, C. (2000). Role of glutathione S-transferases in oxidative stress-induced male germ cell apoptosis. Free Radical Biology and Medicine, 29(10), 1015–1027.
Demasi, A. P., Pereira, G. A., & Netto, L. E. (2006). Yeast oxidative stress response. Influences of cytosolic thioredoxin peroxidase I and of the mitochondrial functional state. FEBS Journal, 273(4), 805–816.
Trachootham, D., Lu, W., Ogasawara, M. A., Valle, N. R. D., & Huang, P. (2008). Redox regulation of cell survival. Antioxidants & Redox Signaling, 10(8), 1343–1374.
Bierben, E., Sahiner, U. M., Sackesen, C., Erzur, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organization Journal, 5(1), 9–19.
Eldh, M., Ekstrom, K., Valadi, H., Sjostrand, M., Olsson, B., Jernas, M., & Lötvall. (2010). Exosomes communicate protective messages during oxidative stress: possible role of exosomal shuttle RNA. PloS One, 5(12), 1–8, e15353.
Lötvall, J., Hill, A. F., Hochberg, F., Buzás, E., Di Vizio, D., Gardiner, C., Gho, Y. S., et al. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 22(3), 26913.
Urbich, C., & Dimmeler, S. (2004). Endothelial progenitor cells functional characterization. Trends in Cardiovascular Medicine, 14(8), 318–322.
Starke, R. D., Ferraro, F., Paschalaki, K. E., Dryden, N. H., Mckinnon, T. A., Sutton, R. E., Payne, E. M., Haskard, D. O., Hughes, A. D., Cutler, D. F., Laffan, M. A., & Randi, A. M. (2011). Endothelial von Willebrand factor regulates angiogenesis. Blood, 117(3), 1071–1080.
Wadhwa, R., Yaguchi, T., Hasan, M. K., Mitsui, Y., Reddel, R. R., & Kaul, S. C. (2002). Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Experimental Cell Research, 274(2), 246–253.
He, T., Peterson, T. E., Holmuhamedov, E. L., Terzic, A., Caplice, M. N., Oberley, L. W., & Katusic, Z. S. (2004). Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(11), 2021–2027.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8(9), 726–762.
Van der Does, A. M., Hensbergen, P. J., Bogaards, S. J., Cansoy, M., Deelder, A. M., van Leeuwen, H. C., Drijfhout, J. W., et al. (2012). The human lactoferrin-derived peptide hLF1-11 exerts immunomodulatory effects by specific inhibition of myeloperoxidase activity. Journal of Immunology, 188(10), 5012–5019.
Picard-Jean, F., Bouchard, S., Larivée, G., & Bisaillon, M. (2014). The intracellular inhibition of HCV replication represents a novel mechanism of action by the innate immune lactoferrin protein. Antiviral Research, 111, 13–22.
Yadav, G., Prasad, R. L., Jha, B. K., Rai, V., Bhakuni, V., & Datta, K. (2009). Evidence for inhibitory interaction of hyaluronan-binding protein 1 (HABP1/p32/gC1qR) with Streptococcus Pneumoniae hyaluronidase. Journal of Biological Chemistry, 284(6), 3897–3905.
Mokarizadeh, A., Delirezh, N., Morshedi, A., Mosayebi, G., Farshid, A. A., & Mardani, K. (2012). Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunology Letters, 147(1–2), 47–54.
Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunology, 15, 1009–1016.
Veevers-Lowe, J., Ball, S. G., Shuttleworth, A., & Kielty, C. M. (2011). Mesenchymal stem cell migration is regulated by fibronectin through alpha 5 beta 1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. Journal of Cell Science, 124, 1288–1300.
Hu, K. X., Sun, Q. Y., Guo, M., & Ai, H. S. (2010). The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. British Journal of Radiology, 83(985), 52–58.
Arslan, F., Lai, R. C., Smeets, M. B., Akeroyd, L., Choo, A., Aguor, E. N., Timmersm, L., Van Rjen, H. V., Doevendans, P. A., Pasterkamp, G., Lim, S. K., & De Kleijn, D. P. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10(3), 301–312.
De Boer, J., Wang, H. J., & Van Blitterswijk, C. (2004). Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Engineering, 10(3–4), 393–401.
Via, A. G., Frizziero, A., & Oliva, F. (2012). Biological properties of mesenchymal stem cells from different sources. Muscles, Ligaments and Tendons Journal, 2(3), 154–162.
Vesiclepedia database. http://www.microvesicles.org/, 2016. Accessed 12 June 2016.
Docheva, D., Haasters, F., & Schieker, M. (2008). Mesenchymal stem cells and their cell surface receptors. Current Rheumatology Reviews, 4, 155–160.
Yoder, M. C. (2012). Human endothelial progenitor cells. Cold Spring Harbor Perspectives in Medicine, 2(7), 1–14, a006692.
Thery, C., Boussac, M., Veron, P., Ricciardi-Castagnoli, P., Raposo, G., Garin, J., & Amigorena, S. (2001). Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. Journal of Immunology, 166(12), 7309–7318.
Choi, D. S., Lee, J. M., Park, G. W., Lim, H. W., Bang, J. Y., Kim, Y. K., Kwon, K. H., et al. (2007). Proteomic analysis of microvesicles derived from human colorectal cancer cells. Journal of Proteome Research, 6(12), 4646–4655.
Hong, B. S., Cho, J. H., Kim, H., Choi, E. J., Rho, S., Kim, J. H., & Choi, D. S. (2009). Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BioMed Central Genomics, 10(556).
Herrera, M. B., Fonsato, V., Gatti, S., Deregibus, M. C., Sordi, A., Cantarella, D., Calogero, R., et al. (2010). Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. Journal of Cellular and Molecular Medicine, 14(6b), 1605–1618.
Kolf, C. M., Cho, E., & Tuan, R. S. (2007). Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, selfrenewal and differentiation. Arthritis Research and Therapy, 9(1), 204.
Gonzales, P. A., Pisitkun, T., Hoffert, J. D., Tchapyjnikov, D., Star, R. A., Kleta, R., Wang, N. S., et al. (2009). Large-scale proteomics and phosphoproteomics of urinary exosomes. Journal of the American Society of Nephrology, 20(2), 363–379.
Looze, C., Yui, D., Leung, L., Ingham, M., Kaler, M., Yao, X., Wu, W. W., et al. (2009). Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochemical and Biophysical Research Communications, 378(3), 433–438.
Mears, R., Craven, R. A., Hanrahan, S., Totty, N., Upton, C., Young, S. L., Patel, P., et al. (2004). Proteomic analysis of melanoma derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics, 4(12), 4019–4031.
Ji, H., Erfani, N., Tauro, B. J., Kapp, E. A., Zhu, H. J., Moritz, R. L., Lim, J. W., & Simpson, R. J. (2008). Difference gel electrophoresis analysis of Ras-transformed fibroblast cell-derived exosomes. Electrophoresis, 29(12), 2660–2671.
Yoder, M. C., & Ingram, D. A. (2009). Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Current Opinion in Hematology, 16, 269–273.
Pasquier, E., & Dias, S. (2010). Endothelial progenitor cells: hope beyond controversy. Current Cancer Drug Targets, 10, 914–921.
Balistreri, C. R., Buffa, S., Pisano, C., Lio, D., Ruvolo, G., & Mazzesi, G. (2015). Are endothelial progenitor cells the real solution for cardiovascular diseases? Focus on controversies and perspectives. BioMed Research International, 2015(2015).
Acknowledgements
We would like to thank all the staff of the Instituto Carlos Chagas (Fiocruz-PR) for the laboratory and administrative support; the staff of the Núcleo de Tecnologia Celular (PUCPR) for tissue collection and the staff of the Electron Microscopy facilities from UFPR and UFSC for image acquisition and help. We thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for use of its facilities, specifically the flow cytometry core facility and mass spectrometry facility. We thank Paulo Moro for the flow cytometry half off set histogram design. This study was supported by Fundação Araucária (Grant number 1005/2013) and MCTI/CNPq/MS (Grant number 404656/2012-9). We thank Capes (Ministério da Educação) for Addeli B.B. Angulski’s fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors report there are no potential conflicts of interest or financial interests.
Electronic supplementary material
Figure S1
Immunophenotypic characterization by flow cytometry of hMSCs. Representative histograms of human bone marrow-derived mesenchymal stem cells with immunophenotypes assessed by flow cytometry. Cells were labeled with fluorescent human antibodies against CD34, CD45, CD19, CD11b, HLA-DR, CD31, CD90, CD105 and CD73. Red histograms indicate the percentage of positive cell populations for each antibody, whereas black histograms indicate isotype-control antibodies. (GIF 66 kb)
Figure S2
Immunophenotypic characterization by flow cytometry of expanded CD133 + cells. Representative histograms of expanded CD133+ cells and immunophenotype assessed by flow cytometry. Cells were labeled with fluorescent antibodies against CD34, CD45, CD14, CD133, CD146, CD31, CD309, CD105 and von Willebrand factor (vWF). Red histograms indicate the percentage of positive cell populations for each antibody, whereas black histograms indicate isotype-control antibodies. (GIF 72 kb)
Figure S3
Apoptosis and necrosis assay with hMSCs and expanded CD133 + cells. (A) (B) (C) Dot plots of three different donors of hMSCs labeled with the PE Annexin V apoptosis detection kit with 7-AAD. (D) (E) (F) Dot plots of three different donors of expanded CD133+ cells labeled with the PE Annexin V apoptosis detection kit with 7-AAD. The dot plots in the lower left corner indicate the live cell population, the dot plots on top indicate the necrotic population (Annexin V and 7-AAD positive), and the dot plots in the lower right corner indicate the apoptotic population. (GIF 44 kb)
Figure S4
Venn diagrams showing an overlap in proteins identified in the four LC-MS/MS analysis. A total of 467 proteins were identified in the two replicates of CD133+-EVs, and 464 proteins were identified in the two replicates of hMSC-EVs. A total of 427 proteins were identified in all MS runs. (GIF 18 kb)
Figure S5
Multi-scatter correlation plot of different replicate runs of EVs derived from expanded CD133 + and hMSC cells. Scatter plots demonstrating the degree of correlation between the 12 different replicate runs (6 replicate runs for CD133+-EVs and 6 replicate runs for hMSC-EVs). The number at the top of each graphic represents the correlation (R square) between the conditions. The correlations are uniformly high and vary only between r = 0.780 and 0.804 among the replicates of the same EV sample and are uniformly low, varying only between r = 0.396 and 0.558 among the replicates of different EV samples. Axis values represents the log 2 (x) value. (GIF 54 kb)
Figure S6
TreeMap visualization of the summary of exclusive GO Biological process for each type of EV obtained by REVIGO analysis. The size of each box is correlated with the frequencies of the occurrence of the GO term. Boxes with the same color are grouped by semantic similarity. (A) and (B) Exclusive GO Biological process for expanded CD133+-EVs and hMSC-EVs, respectively. (GIF 107 kb)
Figure S7
Venn diagram showing the intersections of proteins identified in hMSC-EV samples and the study by Kim and coworkers. A total of 450 proteins overlapped between hMSC-EVs and Kim’s study. (GIF 10 kb)
Figure S8
Comparison of functional enrichment analysis of the hMSC-EV proteome with the study by Kim and coworkers. (A) and (B) Gene ontology enrichment analysis of total proteins identified in hMSC-EVs and Kim and coworkers’ study, showing the most enriched terms for biological processes and molecular function. Pie chart shows some selected significantly enriched categories (p < 0.05). GO analysis was conducted using Funrich software. (GIF 57 kb)
Support Information Table S1
(XLSX 800 kb)
Support Information Table S2
(XLSX 71 kb)
Support Information Table S3
(XLSX 608 kb)
Support Information Table S4
(XLSX 704 kb)
Support Information Table S5
(XLSX 245 kb)
Support Information Table S6
(XLSX 1499 kb)
Support Information Table S7
(XLSX 185 kb)
Rights and permissions
About this article
Cite this article
Angulski, A.B.B., Capriglione, L.G., Batista, M. et al. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why both Sources are Advantageous for Regenerative Medicine. Stem Cell Rev and Rep 13, 244–257 (2017). https://doi.org/10.1007/s12015-016-9715-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9715-z