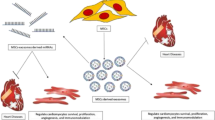
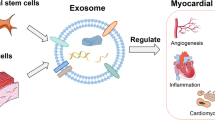
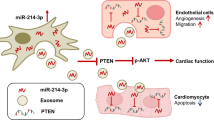
Abstract
Stem cell therapy (SCT) raises the hope for cardiac regeneration in ischemic hearts. However, underlying molecular mechanisms for repair of dead myocardium by SCT in the ischemic heart is poorly understood. Growing evidences suggest that cardiac matrix stiffness and differential expressions of miRNAs play a crucial role in stem cell survival and differentiation. However, their roles on transplanted stem cells, for myocardial repair of the ischemic heart, remain unclear. Transplanted stem cells may act in an autocrine and/or paracrine manner to regenerate the dead myocardium. Paracrine mediators such as stem cell-derived exosomes are emerging as a novel therapeutic strategy to overcome some of the limitations of SCT. These exosomes carry microRNAs (miRNAs) that may regulate stem cell differentiation into a specific lineage. MicroRNAs may also contribute to stiffness of surrounding matrix by regulating extracellular matrix (ECM) turnover. The survival of transplanted stem cell depends on its autophagic process that maintains cellular homeostasis. Therefore, exosomes, miRNAs, extracellular matrix turnover, and autophagy may have an integral role in improving the efficacy of SCT. This review elaborates the specific roles of these regulatory components on cardiac regeneration in the ischemic heart during SCT.




Similar content being viewed by others
References
Li, M., & Izpisua Belmonte, J. C. (2016). Mending a faltering heart. Circulation Research, 118(2), 344–351.
Narula, J., Haider, N., Virmani, R., et al. (1996). Apoptosis in myocytes in end-stage heart failure. The New England Journal of Medicine, 335(16), 1182–1189.
Sanganalmath, S. K., & Bolli, R. (2013). Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circulation Research, 113(6), 810–834.
Anversa, P., Leri, A., Kajstura, J., et al. (2002). Myocyte growth and cardiac repair. Journal of Molecular and Cellular Cardiology, 34(2), 91–105.
Gilbert, P. M., Havenstrite, K. L., Magnusson, K. E., et al. (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science, 329(5995), 1078–1081.
Holst, J., Watson, S., Lord, M. S., et al. (2010). Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nature Biotechnology, 28(10), 1123–1128.
Lei, Y., Gojgini, S., Lam, J., et al. (2011). The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials, 32(1), 39–47.
Mishra, P. K., Chavali, V., Metreveli, N., et al. (2012). Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Canadian Journal of Physiology and Pharmacology, 90(3), 353–360.
Reilly, G. C., & Engler, A. J. (2010). Intrinsic extracellular matrix properties regulate stem cell differentiation. Journal of Biomechanics, 43(1), 55–62.
Shav, D., & Einav, S. (2010). The effect of mechanical loads in the differentiation of precursor cells into mature cells. Annals of the New York Academy of Sciences, 1188, 25–31.
Fingleton, B. (2007). Matrix metalloproteinases as valid clinical targets. Current Pharmaceutical Design, 13(3), 333–346.
Wang, J., Gao, Y., Ma, M., et al. (2013). Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochemistry and Biophysics, 67(2), 537–546.
Chaturvedi, P., Kalani, A., Medina, I., et al. (2015). Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine, 19(9), 2153–2161.
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell, 136(2), 215–233.
Mishra, P. K., Tyagi, N., Kumar, M., et al. (2009). MicroRNAs as a therapeutic target for cardiovascular diseases. Journal of Cellular and Molecular Medicine, 13(4), 778–789.
Nouraee, N., & Mowla, S. J. (2015). miRNA therapeutics in cardiovascular diseases: promises and problems. Frontiers in Genetics, 6, 232.
Wronska, A., Kurkowska-Jastrzebska, I., & Santulli, G. (2015). Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiologica (Oxford, England), 213(1), 60–83.
Maiese, K. (2015). MicroRNAs and SIRT1: a strategy for stem cell renewal and clinical development? Journal of Translational Science, 1(3), 55–57.
Morgado, A. L., Xavier, J. M., Dionisio, P. A., et al. (2015). MicroRNA-34a modulates neural stem cell differentiation by regulating expression of synaptic and autophagic proteins. Molecular Neurobiology, 51(3), 1168–1183.
Levine, B., & Kroemer, G. (2008). Autophagy in the pathogenesis of disease. Cell, 132(1), 27–42.
Purvis, N., Bahn, A., & Katare, R. (2015). The role of MicroRNAs in cardiac stem cells. Stem Cells International, 2015, 194894.
Ratajczak, M. Z., Kucia, M., Jadczyk, T., et al. (2012). Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia, 26(6), 1166–1173.
Zhu, H., & Fan, G. C. (2011). Extracellular/circulating microRNAs and their potential role in cardiovascular disease. American Journal of Cardiovascular Disease, 1(2), 138–149.
Johnstone, R. M. (2005). Revisiting the road to the discovery of exosomes. Blood Cells, Molecules & Diseases, 34(3), 214–219.
Lugini, L., Cecchetti, S., Huber, V., et al. (2012). Immune surveillance properties of human NK cell-derived exosomes. Journal of Immunology, 189(6), 2833–2842.
Mishra, P. K., Singh, S. R., Joshua, I. G., et al. (2010). Stem cells as a therapeutic target for diabetes. Frontiers in Bioscience, 15, 461–477.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Nichols, J., Zevnik, B., Anastassiadis, K., et al. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 95(3), 379–391.
Niwa, H., Burdon, T., Chambers, I., et al. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & Development, 12(13), 2048–2060.
Potten, C. S., Schofield, R., & Lajtha, L. G. (1979). A comparison of cell replacement in bone marrow, testis and three regions of surface epithelium. Biochimica et Biophysica Acta, 560(2), 281–299.
Bernardi, S., Severini, G. M., Zauli, G., et al. (2012). Cell-based therapies for diabetic complications. Experimental Diabetes Research, 2012, 872504.
Leonardini, A., & Avogaro, A. (2013). Abnormalities of the cardiac stem and progenitor cell compartment in experimental and human diabetes. Archives of Physiology and Biochemistry, 119(4), 179–187.
Shen, Y. H., Hu, X., Zou, S., et al. (2012). Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair. The Annals of Thoracic Surgery, 93(5), 1524–1533.
Williams, A. R., & Hare, J. M. (2011). Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research, 109(8), 923–940.
Anversa, P., Kajstura, J., Leri, A., et al. (2006). Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation, 113(11), 1451–1463.
Beltrami, A. P., Barlucchi, L., Torella, D., et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell, 114(6), 763–776.
Hierlihy, A. M., Seale, P., Lobe, C. G., et al. (2002). The post-natal heart contains a myocardial stem cell population. FEBS Letters, 530(1–3), 239–243.
Barile, L., Messina, E., Giacomello, A., et al. (2007). Endogenous cardiac stem cells. Progress in Cardiovascular Diseases, 50(1), 31–48.
Bearzi, C., Rota, M., Hosoda, T., et al. (2007). Human cardiac stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104(35), 14068–14073.
Perez-Moreno, M., Jamora, C., & Fuchs, E. (2003). Sticky business: orchestrating cellular signals at adherens junctions. Cell, 112(4), 535–548.
Urbanek, K., Cesselli, D., Rota, M., et al. (2006). Stem cell niches in the adult mouse heart. Proceedings of the National Academy of Sciences of the United States of America, 103(24), 9226–9231.
Gadue, P., Huber, T. L., Paddison, P. J., et al. (2006). Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 16806–16811.
Lindsley, R. C., Gill, J. G., Murphy, T. L., et al. (2008). Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell, 3(1), 55–68.
Foley, A. C., & Mercola, M. (2005). Heart induction by Wnt antagonists depends on the homeodomain transcription factor hex. Genes & Development, 19(3), 387–396.
Foley, A. C., Korol, O., Timmer, A. M., et al. (2007). Multiple functions of Cerberus cooperate to induce heart downstream of nodal. Developmental Biology, 303(1), 57–65.
Schneider, V. A., & Mercola, M. (2001). Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes & Development, 15(3), 304–315.
Naito, A. T., Shiojima, I., Akazawa, H., et al. (2006). Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 103(52), 19812–19817.
Ueno, S., Weidinger, G., Osugi, T., et al. (2007). Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104(23), 9685–9690.
Qin, G., Ii, M., Silver, M., et al. (2006). Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. The Journal of Experimental Medicine, 203(1), 153–163.
Chen, V. C., Stull, R., Joo, D., et al. (2008). Notch signaling respecifies the hemangioblast to a cardiac fate. Nature Biotechnology, 26(10), 1169–1178.
Rajala, K., Pekkanen-Mattila, M., & Aalto-Setala, K. (2011). Cardiac differentiation of pluripotent stem cells. Stem Cells International, 2011, 383709.
He, Z., Li, H., Zuo, S., et al. (2011). Transduction of Wnt11 promotes mesenchymal stem cell transdifferentiation into cardiac phenotypes. Stem Cells and Development, 20(10), 1771–1778.
Hiroi, Y., Kudoh, S., Monzen, K., et al. (2001). Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nature Genetics, 28(3), 276–280.
Peterkin, T., Gibson, A., & Patient, R. (2003). GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. The EMBO Journal, 22(16), 4260–4273.
Plageman Jr., T. F., & Yutzey, K. E. (2004). Differential expression and function of Tbx5 and Tbx20 in cardiac development. The Journal of Biological Chemistry, 279(18), 19026–19034.
Riley, P., Anson-Cartwright, L., & Cross, J. C. (1998). The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nature Genetics, 18(3), 271–275.
Watt, A. J., Battle, M. A., Li, J., et al. (2004). GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12573–12578.
Marvin, M. J., Di, R. G., Gardiner, A., et al. (2001). Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes & Development, 15(3), 316–327.
Mima, T., Ueno, H., Fischman, D. A., et al. (1995). Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proceedings of the National Academy of Sciences of the United States of America, 92(2), 467–471.
Winnier, G., Blessing, M., Labosky, P. A., et al. (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Development, 9(17), 2105–2116.
Zhang, H., & Bradley, A. (1996). Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development, 122(10), 2977–2986.
Rodolfo, C., Di, B. S., & Cecconi, F. (2016). Autophagy in stem and progenitor cells. Cellular and Molecular Life Sciences, 73(3), 475–496.
Vessoni, A. T., Muotri, A. R., & Okamoto, O. K. (2012). Autophagy in stem cell maintenance and differentiation. Stem Cells and Development, 21(4), 513–520.
Phadwal, K., Watson, A. S., & Simon, A. K. (2013). Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cellular and Molecular Life Sciences, 70(1), 89–103.
Guan, J. L., Simon, A. K., Prescott, M., et al. (2013). Autophagy in stem cells. Autophagy, 9(6), 830–849.
Meng, Y., Ji, J., Tan, W., et al. (2016). Involvement of autophagy in the procedure of endoplasmic reticulum stress introduced apoptosis in bone marrow mesenchymal stem cells from nonobese diabetic mice. Cell Biochemistry and Function, 34(1), 25–33.
Jung, J., Choi, J. H., Lee, Y., et al. (2013). Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. Stem Cells, 31(8), 1584–1596.
Han, Y. F., Sun, T. J., Han, Y. Q., et al. (2015). Clinical perspectives on mesenchymal stem cells promoting wound healing in diabetes mellitus patients by inducing autophagy. European Review for Medical and Pharmacological Sciences, 19(14), 2666–2670.
Ieda, M., Fu, J. D., Delgado-Olguin, P., et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell, 142(3), 375–386.
Nam, Y. J., Song, K., Luo, X., et al. (2013). Reprogramming of human fibroblasts toward a cardiac fate. Proceedings of the National Academy of Sciences of the United States of America, 110(14), 5588–5593.
Wada, R., Muraoka, N., Inagawa, K., et al. (2013). Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proceedings of the National Academy of Sciences of the United States of America, 110(31), 12667–12672.
Fu, J. D., Stone, N. R., Liu, L., et al. (2013). Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports, 1(3), 235–247.
Kaunas, R., Nguyen, P., Usami, S., et al. (2005). Cooperative effects of rho and mechanical stretch on stress fiber organization. Proceedings of the National Academy of Sciences of the United States of America, 102(44), 15895–15900.
Kurpinski, K., Chu, J., Hashi, C., et al. (2006). Anisotropic mechanosensing by mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16095–16100.
Kurpinski, K., Park, J., Thakar, R. G., et al. (2006). Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. Molecular & Cellular Biomechanics, 3(1), 21–34.
Park, J. S., Chu, J. S., Cheng, C., et al. (2004). Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnology and Bioengineering, 88(3), 359–368.
Kurpinski, K., Lam, H., Chu, J., et al. (2010). Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells, 28(4), 734–742.
Wang, D., Park, J. S., Chu, J. S., et al. (2004). Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. The Journal of Biological Chemistry, 279(42), 43725–43734.
Park, J. S., Chu, J. S., Tsou, A. D., et al. (2011). The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials, 32(16), 3921–3930.
Tyagi, S. C., & Hoit, B. D. (2002). Metalloproteinase in myocardial adaptation and maladaptation. Journal of Cardiovascular Pharmacology and Therapeutics, 7(4), 241–246.
Ali, M. A., & Schulz, R. (2009). Activation of MMP-2 as a key event in oxidative stress injury to the heart. Frontiers in Bioscience, 14, 699–716.
Mishra, P. K., Givvimani, S., Chavali, V., et al. (2013). Cardiac matrix: a clue for future therapy. Biochimica et Biophysica Acta, 1832(12), 2271–2276.
Morancho, A., Ma, F., Barcelo, V., et al. (2015). Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 35(10), 1547–1551.
Guo, J., Jie, W., Shen, Z., et al. (2014). SCF increases cardiac stem cell migration through PI3K/AKT and MMP2/9 signaling. International Journal of Molecular Medicine, 34(1), 112–118.
Pottier, N., Cauffiez, C., Perrais, M., et al. (2014). FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends in Pharmacological Sciences, 35(3), 119–126.
Tyagi, A. C., Sen, U., & Mishra, P. K. (2011). Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Current Diabetes Reviews, 7(6), 367–376.
Callis, T. E., Deng, Z., Chen, J. F., et al. (2008). Muscling through the microRNA world. Experimental Biology and Medicine (Maywood), 233(2), 131–138.
van, R. E., Sutherland, L. B., Thatcher, J. E., et al. (2008). Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13027–13032.
Lee, Y., Yang, X., Huang, Y., et al. (2010). Network modeling identifies molecular functions targeted by miR-204 to suppress head and neck tumor metastasis. PLoS Computational Biology, 6(4), e1000730.
Ucar, A., Vafaizadeh, V., Jarry, H., et al. (2010). miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nature Genetics, 42(12), 1101–1108.
Bronisz, A., Godlewski, J., Wallace, J. A., et al. (2012). Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nature Cell Biology, 14(2), 159–167.
Nan, Y., Han, L., Zhang, A., et al. (2010). MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Research, 1359, 14–21.
Yan, W., Zhang, W., Sun, L., et al. (2011). Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Research, 1411, 108–115.
Huang, X. H., Chen, J. S., Wang, Q., et al. (2011). miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. The Journal of Pathology, 225(3), 463–472.
Moriyama, T., Ohuchida, K., Mizumoto, K., et al. (2009). MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Molecular Cancer Therapeutics, 8(5), 1067–1074.
Rossi, M., Pitari, M. R., Amodio, N., et al. (2013). miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J. Cellular Physiology, 228(7), 1506–1515.
Tavazoie, S. F., Alarcon, C., Oskarsson, T., et al. (2008). Endogenous human microRNAs that suppress breast cancer metastasis. Nature, 451(7175), 147–152.
Yang, F., Yin, Y., Wang, F., et al. (2010). miR-17-5p promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology, 51(5), 1614–1623.
Felli, N., Felicetti, F., Lustri, A. M., et al. (2013). miR-126&126* restored expressions play a tumor suppressor role by directly regulating ADAM9 and MMP7 in melanoma. Plos One, 8(2), e56824.
Kano, M., Seki, N., Kikkawa, N., et al. (2010). miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. INT. J. Cancer, 127(12), 2804–2814.
Liu, X., Yu, J., Jiang, L., et al. (2009). MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics & Proteomics, 6(3), 131–139.
Stanczyk, J., Ospelt, C., Karouzakis, E., et al. (2011). Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis and Rheumatism, 63(2), 373–381.
Jones, S. W., Watkins, G., Le, G. N., et al. (2009). The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis and Cartilage, 17(4), 464–472.
Henson, B. J., Bhattacharjee, S., O'Dee, D. M., et al. (2009). Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes, Chromosomes & Cancer, 48(7), 569–582.
Akhtar, N., Rasheed, Z., Ramamurthy, S., et al. (2010). MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis and Rheumatism, 62(5), 1361–1371.
Tardif, G., Hum, D., Pelletier, J. P., et al. (2009). Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskeletal Disorders, 10, 148.
Xu, N., Zhang, L., Meisgen, F., et al. (2012). MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. The Journal of Biological Chemistry, 287(35), 29899–29908.
Osaki, M., Takeshita, F., Sugimoto, Y., et al. (2011). MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Molecular Therapy, 19(6), 1123–1130.
Grimson, A., Farh, K. K., Johnston, W. K., et al. (2007). MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular Cell, 27(1), 91–105.
Fornari, F., Milazzo, M., Chieco, P., et al. (2012). In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. The Journal of Pathology, 227(3), 275–285.
Gennarino, V. A., Sardiello, M., Avellino, R., et al. (2009). MicroRNA target prediction by expression analysis of host genes. Genome Research, 19(3), 481–490.
Chuang, T. D., Panda, H., Luo, X., et al. (2012). miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocrine-Related Cancer, 19(4), 541–556.
Wang, B., Hsu, S. H., Majumder, S., et al. (2010). TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene, 29(12), 1787–1797.
Limana, F., Esposito, G., D'Arcangelo, D., et al. (2011). HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PloS One, 6(6), e19845.
Chi, S. W., Zang, J. B., Mele, A., et al. (2009). Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature, 460(7254), 479–486.
Yu, J. Y., Chung, K. H., Deo, M., et al. (2008). MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Experimental Cell Research, 314(14), 2618–2633.
Lu, Y., Roy, S., Nuovo, G., et al. (2011). Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamoxifen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. The Journal of Biological Chemistry, 286(49), 42292–42302.
Zhang, A., Liu, Y., Shen, Y., et al. (2011). miR-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology, 78(2), 474–479.
Baek, D., Villen, J., Shin, C., et al. (2008). The impact of microRNAs on protein output. Nature, 455(7209), 64–71.
Zhang, C., Zhang, J., Hao, J., et al. (2012). High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. Journal of Translational Medicine, 10, 119.
Yu, D., Zhou, H., Xun, Q., et al. (2012). microRNA-103 regulates the growth and invasion of endometrial cancer cells through the downregulation of tissue inhibitor of metalloproteinase 3. Oncology Letters, 3(6), 1221–1226.
Helwak, A., Kudla, G., Dudnakova, T., et al. (2013). Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell, 153(3), 654–665.
Luna, C., Li, G., Qiu, J., et al. (2011). MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. Journal of Cellular Physiology, 226(5), 1407–1414.
Martin, J., Jenkins, R. H., Bennagi, R., et al. (2011). Post-transcriptional regulation of transforming growth factor Beta-1 by microRNA-744. PloS One, 6(10), e25044.
Tili, E., Michaille, J. J., Alder, H., et al. (2010). Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochemical Pharmacology, 80(12), 2057–2065.
Liu, Z. Y., Zhang, G. L., Wang, M. M., et al. (2011). MicroRNA-663 targets TGFB1 and regulates lung cancer proliferation. Asian Pacific Journal of Cancer Prevention, 12(11), 2819–2823.
Gabriely, G., Wurdinger, T., Kesari, S., et al. (2008). MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Molecular and Cellular Biology, 28(17), 5369–5380.
Tsai, W. C., Hsu, S. D., Hsu, C. S., et al. (2012). MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. The Journal of Clinical Investigation, 122(8), 2884–2897.
Lv, X. B., Jiao, Y., Qing, Y., et al. (2011). miR-124 suppresses multiple steps of breast cancer metastasis by targeting a cohort of pro-metastatic genes in vitro. CHIN J. Cancer, 30(12), 821–830.
Ernst, A., Campos, B., Meier, J., et al. (2010). De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene, 29(23), 3411–3422.
Sander, S., Bullinger, L., Klapproth, K., et al. (2008). MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood, 112(10), 4202–4212.
Xie, H., Zhao, Y., Caramuta, S., et al. (2012). miR-205 expression promotes cell proliferation and migration of human cervical cancer cells. Plos One, 7(10), e46990.
Lee, H. K., Bier, A., Cazacu, S., et al. (2013). MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PloS One, 8(2), e54652.
Tsukamoto, Y., Nakada, C., Noguchi, T., et al. (2010). MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Research, 70(6), 2339–2349.
Pichiorri, F., Suh, S. S., Ladetto, M., et al. (2008). MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 12885–12890.
Takaya, T., Ono, K., Kawamura, T., et al. (2009). MicroRNA-1 and MicroRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circulation Journal, 73(8), 1492–1497.
Ivey, K. N., Muth, A., Arnold, J., et al. (2008). MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell, 2(3), 219–229.
Kuppusamy, K. T., Sperber, H., & Ruohola-Baker, H. (2013). MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. Current Molecular Medicine, 13(5), 757–764.
Seeger, F. H., Zeiher, A. M., & Dimmeler, S. (2013). MicroRNAs in stem cell function and regenerative therapy of the heart. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(8), 1739–1746.
Kane, N. M., Thrasher, A. J., Angelini, G. D., et al. (2014). Concise review: MicroRNAs as modulators of stem cells and angiogenesis. Stem Cells, 32(5), 1059–1066.
Wilson, K. D., Hu, S., Venkatasubrahmanyam, S., et al. (2010). Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circulation. Cardiovascular Genetics, 3(5), 426–435.
Bras-Rosario, L., Matsuda, A., Pinheiro, A. I., et al. (2013). Expression profile of microRNAs regulating proliferation and differentiation in mouse adult cardiac stem cells. PloS One, 8(5), e63041.
Luna, C., Li, G., Qiu, J., et al. (2011). Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Investigative Ophthalmology & Visual Science, 52(6), 3567–3572.
Jayawardena, T. M., Egemnazarov, B., Finch, E. A., et al. (2012). MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circulation Research, 110(11), 1465–1473.
Abdelwahid, E., Siminiak, T., Guarita-Souza, L. C., et al. (2011). Stem cell therapy in heart diseases: a review of selected new perspectives, practical considerations and clinical applications. Current Cardiology Reviews, 7(3), 201–212.
Passier, R., van Laake, L. W., & Mummery, C. L. (2008). Stem-cell-based therapy and lessons from the heart. Nature, 453(7193), 322–329.
Wu, X., Ding, S., Ding, Q., et al. (2004). Small molecules that induce cardiomyogenesis in embryonic stem cells. Journal of the American Chemical Society, 126(6), 1590–1591.
Zhang, J., Wilson, G. F., Soerens, A. G., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research, 104(4), e30–e41.
Strauer, B. E., Brehm, M., Zeus, T., et al. (2002). Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation, 106(15), 1913–1918.
Orlic, D., Kajstura, J., Chimenti, S., et al. (2001). Transplanted adult bone marrow cells repair myocardial infarcts in mice. Annals of the New York Academy of Sciences, 938, 221–229.
Schuleri, K. H., Feigenbaum, G. S., Centola, M., et al. (2009). Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. European Heart Journal, 30(22), 2722–2732.
Jackson, K. A., Majka, S. M., Wang, H., et al. (2001). Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation, 107(11), 1395–1402.
Gomez-Mauricio, R. G., Acarregui, A., Sanchez-Margallo, F. M., et al. (2013). A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. European Journal of Pharmaceutics and Biopharmaceutics, 84(1), 29–39.
Badorff, C., Brandes, R. P., Popp, R., et al. (2003). Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation, 107(7), 1024–1032.
Rupp, S., Badorff, C., Koyanagi, M., et al. (2004). Statin therapy in patients with coronary artery disease improves the impaired endothelial progenitor cell differentiation into cardiomyogenic cells. Basic Research in Cardiology, 99(1), 61–68.
Tang, X. L., Rokosh, G., Sanganalmath, S. K., et al. (2010). Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation, 121(2), 293–305.
Gnecchi, M., He, H., Noiseux, N., et al. (2006). Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal, 20(6), 661–669.
Haider, H. K., Jiang, S., Idris, N. M., et al. (2008). IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circulation Research, 103(11), 1300–1308.
Sahoo, S., Klychko, E., Thorne, T., et al. (2011). Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation Research, 109(7), 724–728.
Han, C., Sun, X., Liu, L., et al. (2016). Exosomes and their therapeutic potentials of stem cells. Stem Cells International, 2016, 7653489.
Stoorvogel, W., Strous, G. J., Geuze, H. J., et al. (1991). Late endosomes derive from early endosomes by maturation. Cell, 65(3), 417–427.
Kishore, R., & Khan, M. (2016). More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circulation Research, 118(2), 330–343.
Pan, B. T., & Johnstone, R. M. (1983). Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell, 33(3), 967–978.
Lai, R. C., Arslan, F., Lee, M. M., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222.
Vrijsen, K. R., Sluijter, J. P., Schuchardt, M. W., et al. (2010). Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. Journal of Cellular and Molecular Medicine, 14(5), 1064–1070.
Raposo, G., Nijman, H. W., Stoorvogel, W., et al. (1996). B lymphocytes secrete antigen-presenting vesicles. The Journal of Experimental Medicine, 183(3), 1161–1172.
Peters, P. J., Geuze, H. J., van der Donk, H. A., et al. (1989). Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. European Journal of Immunology, 19(8), 1469–1475.
Zitvogel, L., Regnault, A., Lozier, A., et al. (1998). Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Medicine, 4(5), 594–600.
Heijnen, H. F., Schiel, A. E., Fijnheer, R., et al. (1999). Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood, 94(11), 3791–3799.
Fevrier, B., Vilette, D., Archer, F., et al. (2004). Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America, 101(26), 9683–9688.
Dignat-George, F., & Boulanger, C. M. (2011). The many faces of endothelial microparticles. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(1), 27–33.
Wolfers, J., Lozier, A., Raposo, G., et al. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Medicine, 7(3), 297–303.
Simons, M., & Raposo, G. (2009). Exosomes--vesicular carriers for intercellular communication. Current Opinion in Cell Biology, 21(4), 575–581.
Mittelbrunn, M., Gutierrez-Vazquez, C., Villarroya-Beltri, C., et al. (2011). Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications, 2, 282.
Dai, S., Wan, T., Wang, B., et al. (2005). More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clinical Cancer Research, 11(20), 7554–7563.
Korkut, C., Ataman, B., Ramachandran, P., et al. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 139(2), 393–404.
Soderberg, A., Barral, A. M., Soderstrom, M., et al. (2007). Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radical Biology & Medicine, 43(1), 90–99.
Chistiakov, D.A., Orekhov, A.N., Bobryshev, Y.V. (2016). Cardiac extracellular vesicles in normal and infarcted heart. International Journal of Molecular Sciences, 17(1), 63–81.
Garcia, N. A., Moncayo-Arlandi, J., Sepulveda, P., et al. (2015). Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovascular Research, 109(3), 397–408.
Khan, M., Nickoloff, E., Abramova, T., et al. (2015). Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation Research, 117(1), 52–64.
Chen, L., Wang, Y., Pan, Y., et al. (2013). Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochemical and Biophysical Research Communications, 431(3), 566–571.
Gray, W. D., French, K. M., Ghosh-Choudhary, S., et al. (2015). Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation Research, 116(2), 255–263.
Ibrahim, A. G., Cheng, K., & Marban, E. (2014). Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports, 2(5), 606–619.
Wang, J., Huang, W., Xu, R., et al. (2012). MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. Journal of Cellular and Molecular Medicine, 16(9), 2150–2160.
Gurha, P., Abreu-Goodger, C., Wang, T., et al. (2012). Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation, 125(22), 2751–2761.
Lyu, L., Wang, H., Li, B., et al. (2015). A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. Journal of Molecular and Cellular Cardiology, 89(Pt B), 268–279.
Wang, X., Huang, W., Liu, G., et al. (2014). Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of Molecular and Cellular Cardiology, 74, 139–150.
Behfar, A., Crespo-Diaz, R., Terzic, A., et al. (2014). Cell therapy for cardiac repair--lessons from clinical trials. Nature Reviews. Cardiology, 11(4), 232–246.
van Berlo, J. H., Kanisicak, O., Maillet, M., et al. (2014). C-kit + cells minimally contribute cardiomyocytes to the heart. Nature, 509(7500), 337–341.
Sultana, N., Zhang, L., Yan, J., et al. (2015). Resident c-kit(+) cells in the heart are not cardiac stem cells. Nature Communications, 6, 8701.
Ilic, D., Devito, L., Miere, C., & Codognotto, S. (2015). Human embryonic and induced pluripotent stem cells in clinical trials. British Medical Bulletin, 116, 19–27.
Tarui, S., Ishigami, S., Ousaka, D., et al. (2015). Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: three-year follow-up of the Transcoronary infusion of cardiac progenitor cells in patients with single-ventricle physiology (TICAP) trial. The Journal of Thoracic and Cardiovascular Surgery, 150(5), 1198–1207, 1208.
Poulin, M.F., Deka, A., Mohamedali, B., et al. (2016). Clinical benefits of stem cells for chronic symptomatic systolic heart failure a systematic review of the existing data and ongoing trials. Cell Transplantation. doi:10.3727/096368916X692087.
Banovic, M., Loncar, Z., Behfar, A., et al. (2015). Endpoints in stem cell trials in ischemic heart failure. Stem Cell Research & Therapy, 6, 159.
Oh, H., Ito, H., Sano, S. (2016) Challenges to success in heart failure: cardiac cell therapies in patients with heart diseases. Journal of Cardiology, 68(5), 361–367.
Micheu, M. M., Scafa-Udriste, A., & DorobanTu, M. (2016). Bringing cardiac stem cell therapy from bench to bedside: lessons from the past and future perspectives. Romanian Journal of Morphology and Embryology, 57(2), 367–372.
Bruyneel, A.A., Sehgal, A., Malandraki-Miller, S., et al. (2016) Stem cell therapy for the heart: blind alley or magic bullet? Journal of Cardiovascular Translational Research. PMID 27542008, doi:10.1007/s12265-016-9708-y.
Ong, S. G., Lee, W. H., Huang, M., et al. (2014). Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation, 130(11 Suppl 1), S60–S69.
Mackie, A. R., Klyachko, E., Thorne, T., et al. (2012). Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circulation Research, 111(3), 312–321.
Akyurekli, C., Le, Y., Richardson, R. B., et al. (2015). A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Reviews, 11(1), 150–160.
Singla, D. K. (2016). Stem cells and exosomes in cardiac repair. Current Opinion in Pharmacology, 27, 19–23.
Spinetti, G., Fortunato, O., Caporali, A., et al. (2013). MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circulation Research, 112(2), 335–346.
Janssen, H. L., Reesink, H. W., Lawitz, E. J., et al. (2013). Treatment of HCV infection by targeting microRNA. The New England Journal of Medicine, 368(18), 1685–1694.
Das, S., & Halushka, M. K. (2015). Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovascular Pathology, 24(4), 199–206.
Lin, Z., & Pu, W. T. (2014). Strategies for cardiac regeneration and repair. Science Translational Medicine, 6(239), 239rv1.
Acknowledgments
This work was supported in part by the National Institutes of Health grants: HL-113281 and HL-116205 to Paras Kumar Mishra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors confirm that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Prathipati, P., Nandi, S.S. & Mishra, P.K. Stem Cell-Derived Exosomes, Autophagy, Extracellular Matrix Turnover, and miRNAs in Cardiac Regeneration during Stem Cell Therapy. Stem Cell Rev and Rep 13, 79–91 (2017). https://doi.org/10.1007/s12015-016-9696-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9696-y