Abstract
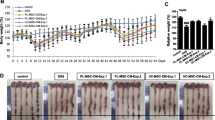
Inflammatory bowel diseases (IBDs) are the result of pathological immune responses due to environmental factors or microbial antigens into a genetically predisposed individual. Mainly due to their trophic properties, a mounting interest is focused on the use of human mesenchymal stem/stromal cells (hMSCs) to treat IBD disease in animal models. The aim of the study is to test whether the secreted molecules, derived from a specific population of second trimester amniotic fluid mesenchymal stem/stromal cells, the spindle-shaped MSCs (SS-AF-MSCs), could be utilized as a novel therapeutic, cell free approach for IBD therapy. Induction of colitis was achieved by oral administration of dextran sulphate sodium (DSS) (3 % w/v in tap water), for 5 days, to 8-week-old NOD/SCID mice. The progression of colitis was assessed on a daily basis through recording the body weight, stool consistency and bleeding. Conditioned media (CM) derived from SS-AF-MSCs were collected, concentrated and then delivered intraperitoneally into DSS treated mice. To evaluate and determine the inflammatory cytokine levels, histopathological approach was applied. Administration of CM derived from SS-AF-MSCs cells reduced the severity of colitis in mice. More importantly, TGFb1 protein levels were increased in the mice received CM, while TNFa and MMP2 protein levels were decreased, respectively. Accordingly, IL-10 was significantly increased in mice received CM, whereas TNFa and IL-1b were decreased at mRNA level. Our results demonstrated that CM derived from SS-AF-MSCs cells is able to ameliorate DSS-induced colitis in immunodeficient colitis mouse model, and thus, it has a potential for use in IBD therapy.





Similar content being viewed by others
References
Cho, J. H. (2008). The genetics and immunopathogenesis of inflammatory bowel disease. Nature Reviews Immunology, 8(6), 458–466. doi:10.1038/nri2340.
Rogler, G., Vavricka, S., Schoepfer, A., & Lakatos, P. L. (2013). Mucosal healing and deep remission: what does it mean? World Journal of Gastroenterology, 19(43), 7552–7560. doi:10.3748/wjg.v19.i43.7552.
Hommes, D., & Travis, S. (2012). Leading change in inflammatory bowel disease. Foreword. Journal of Crohn's & Colitis, 6(Suppl 2), S223. doi:10.1016/S1873-9946(12)60501-7.
Thomas, A., & Lodhia, N. (2014). Advanced therapy for inflammatory bowel disease: a guide for the primary care physician. Journal of the American Board of Family Medicine : JABFM, 27(3), 411–420. doi:10.3122/jabfm.2014.03.130224.
Peyrin-Biroulet, L., Loftus Jr., E. V., Colombel, J. F., & Sandborn, W. J. (2011). Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflammatory Bowel Diseases, 17(1), 471–478. doi:10.1002/ibd.21417.
Jneid, H., Anderson, J.L., Wright, R.S., Adams, C.D., Bridges, C.R., Casey, D.E., Jr., Ettinger, S.M., Fesmire, F.M., Ganiats, T.G,, Lincoff, A.M., Peterson, E.D., Philippides, G.J., Theroux, P., Wenger, N.K., Zidar, J.P. (2012) 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Journal of the American College of Cardiology 60 (7), 645–681. doi:10.1016/j.jacc.2012.06.004
Desreumaux, P., Foussat, A., Allez, M., Beaugerie, L., Hebuterne, X., Bouhnik, Y., Nachury, M., Brun, V., Bastian, H., Belmonte, N., Ticchioni, M., Duchange, A., Morel-Mandrino, P., Neveu, V., Clerget-Chossat, N., Forte, M., Colombel, J.F., (2012) Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143 (5), 1207–1217–e1201–1202. doi:10.1053/j.gastro.2012.07.116
Thomson, A. W., & Robbins, P. D. (2008). Tolerogenic dendritic cells for autoimmune disease and transplantation. Annals of the Rheumatic Diseases, 67(Suppl 3), iii90–iii96. doi:10.1136/ard.2008.099176.
Irhimeh, M. R., & Cooney, J. (2016). Management of inflammatory bowel disease using stem cell therapy. Current Stem Cell Research & Therapy, 11, 72–77.
Park, J. S., Yi, T. G., Park, J. M., Han, Y. M., Kim, J. H., Shin, D. H., Tak, S. J., Lee, K., Lee, Y. S., Jeon, M. S., Hahm, K. B., Song, S. U., & Park, S. H. (2015). Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. Journal of Clinical Biochemistry and Nutrition, 57(3), 192–203. doi:10.3164/jcbn.15-56.
Forte, D., Ciciarello, M., Valerii, M. C., De Fazio, L., Cavazza, E., Giordano, R., Parazzi, V., Lazzari, L., Laureti, S., Rizzello, F., Cavo, M., Curti, A., Lemoli, R. M., Spisni, E., & Catani, L. (2015). Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn’s disease patients in a mouse model of colitis. Stem Cell Research & Therapy, 6, 170. doi:10.1186/s13287-015-0166-2.
Perez-Merino, E. M., Uson-Casaus, J. M., Zaragoza-Bayle, C., Duque-Carrasco, J., Marinas-Pardo, L., Hermida-Prieto, M., Barrera-Chacon, R., & Gualtieri, M. (2015). Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: clinical and laboratory outcomes. Veterinary Journal. doi:10.1016/j.tvjl.2015.08.003.
Uccelli, A., Moretta, L., & Pistoia, V. (2006). Immunoregulatory function of mesenchymal stem cells. European Journal of Immunology, 36(10), 2566–2573. doi:10.1002/eji.200636416.
Duijvestein, M., Vos, A. C., Roelofs, H., Wildenberg, M. E., Wendrich, B. B., Verspaget, H. W., Kooy-Winkelaar, E. M., Koning, F., Zwaginga, J. J., Fidder, H. H., Verhaar, A. P., Fibbe, W. E., van den Brink, G. R., & Hommes, D. W. (2010). Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut, 59(12), 1662–1669. doi:10.1136/gut.2010.215152.
Touboul, T., Hannan, N. R., Corbineau, S., Martinez, A., Martinet, C., Branchereau, S., Mainot, S., Strick-Marchand, H., Pedersen, R., Di Santo, J., Weber, A., & Vallier, L. (2010). Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology, 51(5), 1754–1765. doi:10.1002/hep.23506.
Martinez-Montiel, M. P., Gomez-Gomez, G. J., & Flores, A. I. (2014). Therapy with stem cells in inflammatory bowel disease. World Journal of Gastroenterology, 20(5), 1211–1227. doi:10.3748/wjg.v20.i5.1211.
Kim, N., & Cho, S. G. (2015). New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. International Journal of stem cells, 8(1), 54–68. doi:10.15283/ijsc.2015.8.1.54.
Trohatou, O., Anagnou, N. P., & Roubelakis, M. G. (2013). Human amniotic fluid stem cells as an attractive tool for clinical applications. Current Stem Cell Research & Therapy, 8(2), 125–132.
Roubelakis, M. G. (2013). Therapeutic potential of fetal mesenchymal stem cells. Current Stem Cell Research & Therapy, 8(2), 115–116.
Roubelakis, M. G., Bitsika, V., Zagoura, D., Trohatou, O., Pappa, K. I., Makridakis, M., Antsaklis, A., Vlahou, A., & Anagnou, N. P. (2011). In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. Journal of Cellular and Molecular Medicine, 15(9), 1896–1913. doi:10.1111/j.1582-4934.2010.01180.x.
Roubelakis, M. G., Pappa, K. I., Bitsika, V., Zagoura, D., Vlahou, A., Papadaki, H. A., Antsaklis, A., & Anagnou, N. P. (2007). Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells and Development, 16(6), 931–952. doi:10.1089/scd.2007.0036.
Zagoura, D. S., Trohatou, O., Bitsika, V., Makridakis, M., Pappa, K. I., Vlahou, A., Roubelakis, M. G., & Anagnou, N. P. (2013). AF-MSCs fate can be regulated by culture conditions. Cell Death & Disease, 4, e571. doi:10.1038/cddis.2013.93.
Zagoura, D. S., Roubelakis, M. G., Bitsika, V., Trohatou, O., Pappa, K. I., Kapelouzou, A., Antsaklis, A., & Anagnou, N. P. (2012). Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut, 61(6), 894–906. doi:10.1136/gutjnl-2011-300908.
Roubelakis, M. G., Tsaknakis, G., Pappa, K. I., Anagnou, N. P., & Watt, S. M. (2013). Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PloS One, 8(1), e54747. doi:10.1371/journal.pone.0054747.
Watanabe, S., Arimura, Y., Nagaishi, K., Isshiki, H., Onodera, K., Nasuno, M., Yamashita, K., Idogawa, M., Naishiro, Y., Murata, M., Adachi, Y., Fujimiya, M., Imai, K., & Shinomura, Y. (2014). Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. Journal of Gastroenterology, 49(2), 270–282. doi:10.1007/s00535-013-0901-3.
Whittem, C. G., Williams, A. D., & Williams, C. S. (2010). Murine colitis modeling using dextran sulfate sodium (DSS). Journal of visualized experiments : JoVE, 35. doi:10.3791/1652.
Zhang, H., Zhang, X., Ding, X., Cao, W., Qu, L., & Zhou, G. (2014). Effect of secondary lymphoid tissue chemokine suppression on experimental ulcerative colitis in mice. Genetics and molecular research : GMR, 13(2), 3337–3345. doi:10.4238/2014.April.29.12.
Remmele, W., & Stegner, H. E. (1987). Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathologe, 8(3), 138–140.
De Fazio, L., Cavazza, E., Spisni, E., Strillacci, A., Centanni, M., Candela, M., Pratico, C., Campieri, M., Ricci, C., & Valerii, M. C. (2014). Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World Journal of Gastroenterology, 20(8), 2051–2061. doi:10.3748/wjg.v20.i8.2051.
Pedersen, G. (2015). Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium. Danish medical journal, 62(1), B4973.
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822. doi:10.1182/blood-2004-04-1559.
Prockop, D. J. (2009). Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Molecular therapy : the journal of the American Society of Gene Therapy, 17(6), 939–946. doi:10.1038/mt.2009.62.
He, X. W., He, X. S., Lian, L., Wu, X. J., & Lan, P. (2012). Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Digestive Diseases and Sciences, 57(12), 3136–3144. doi:10.1007/s10620-012-2290-5.
Wei, Y., Nie, Y., Lai, J., Wan, Y. J., & Li, Y. (2009). Comparison of the population capacity of hematopoietic and mesenchymal stem cells in experimental colitis rat model. Transplantation, 88(1), 42–48. doi:10.1097/TP.0b013e3181a9f0a7.
Khalil, P. N., Weiler, V., Nelson, P. J., Khalil, M. N., Moosmann, S., Mutschler, W. E., Siebeck, M., & Huss, R. (2007). Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology, 132(3), 944–954. doi:10.1053/j.gastro.2006.12.029.
Uccelli, A., Laroni, A., Freedman, M.S., (2011) Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. The Lancet Neurology 10 (7), 649–656. doi:10.1016/S1474-4422(11)70121-1
Dionne, S., Hiscott, J., D’Agata, I., Duhaime, A., & Seidman, E. G. (1997). Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Digestive Diseases and Sciences, 42(7), 1557–1566.
Kucharzik, T., Stoll, R., Lugering, N., & Domschke, W. (1995). Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clinical and Experimental Immunology, 100(3), 452–456.
Chen, Z., He, X., He, X., Chen, X., Lin, X., Zou, Y., Wu, X., & Lan, P. (2014). Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochemical and Biophysical Research Communications, 450(4), 1402–1408. doi:10.1016/j.bbrc.2014.07.002.
Telgenhoff, D., & Shroot, B. (2005). Cellular senescence mechanisms in chronic wound healing. Cell Death and Differentiation, 12(7), 695–698. doi:10.1038/sj.cdd.4401632.
Mao, J. W., Tang, H. Y., Tan, X. Y., & Wang, Y. D. (2012). Effect of Etiasa on the expression of matrix metalloproteinase-2 and tumor necrosis factor-alpha in a rat model of ulcerative colitis. Molecular Medicine Reports, 6(5), 996–1000. doi:10.3892/mmr.2012.1021.
O’Sullivan, S., Gilmer, J. F., & Medina, C. (2015). Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators of Inflammation, 2015, 964131. doi:10.1155/2015/964131.
Del Zotto, B., Mumolo, G., Pronio, A. M., Montesani, C., Tersigni, R., & Boirivant, M. (2003). TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn’s disease and ulcerative colitis. Clinical and Experimental Immunology, 134(1), 120–126.
Liu, W., Zhang, S., Gu, S., Sang, L., & Dai, C. (2015). Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFbeta1. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 35(3), 858–865. doi:10.1159/000369743.
Delcenserie, V., Martel, D., Lamoureux, M., Amiot, J., Boutin, Y., & Roy, D. (2008). Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology, 10(1–2), 37–54.
Chen, Q. Q., Yan, L., Wang, C. Z., Wang, W. H., Shi, H., Su, B. B., Zeng, Q. H., Du, H. T., & Wan, J. (2013). Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World Journal of Gastroenterology, 19(29), 4702–4717. doi:10.3748/wjg.v19.i29.4702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Legaki, E., Roubelakis, M.G., Theodoropoulos, G.E. et al. Therapeutic Potential of Secreted Molecules Derived from Human Amniotic Fluid Mesenchymal Stem/Stroma Cells in a Mice Model of Colitis. Stem Cell Rev and Rep 12, 604–612 (2016). https://doi.org/10.1007/s12015-016-9677-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9677-1