Abstract
Human embryonic stem cells (hESCs) have the potential to differentiate into all cells of the three germ layers, thus making them an attractive source of cells for use in regenerative medicine. The greatest challenge lies in regulating the differentiation of hESCs into specific cell lineages by both intrinsic and extrinsic factors. In this study we determined the effect of a fibroblast-derived extracellular matrix (fd-ECM) on hESCs differentiation. We demonstrate that growth of hESCs on fd-ECM results in hESCs losing their stemness and proliferation potential. As the stem cells differentiate they attain gene expression profiles similar to the primitive streak of the in vivo embryo. The activation of both the MEK-ERK and Wnt/β-catenin signaling pathways is required for the fd-ECM-mediated differentiation of hESCs towards the endoderm and involves integrins α1, α2, α3 and β1. This study illustrates the importance of the cellular microenvironment in directing stem cell fate and that the nature and composition of the extracellular matrix is a crucial determining factor.








Similar content being viewed by others
References
Evans, M. J., & Kaufmann, M. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Nakagawa, M., Koyanagi, M., Tanabe, K., et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology, 26(1), 101–106.
McNeish, J. (2004). Embryonic stem cells in drug discovery. Nature Reviews Drug Discovery, 3(1), 70–80.
Mehta, A., Bhaskar, V., Konala, R., Khanna, A., & Majumdar, A. S. (2008). Assessment of drug induced developmental toxicity using human embryonic stem cells. Cell Biology International, 32(11), 1412–1424.
Nichols, J., Zevnik, B., Anastassiadis, K., et al. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct 4. Cell, 95(3), 379–391.
Avilion, A. A., Nicolis, S. K., Pevny, L. H., Perez, L., Vivian, N., & Lovell-Badge, R. (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes and Development, 17(1), 126–140.
Jaenisch, R., & Young, R. (2008). Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell, 132(4), 567–582.
Arnold, S. J., & Robertson, E. J. (2009). Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nature Reviews Molecular Cell Biology, 10(2), 91–103.
Evans, N. D., Gentleman, E., Chen, X., Roberts, C. J., Polak, J. M., & Stevens, M. M. (2010). Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials, 31(12), 3244–3252.
Oberwallner, B., Brodarac, A., Anic, P., et al. (2014). Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. European Journal of Cardio-Thoracic Surgery. doi:10.1093/ejcts/ezu163. 1–10.
Czyz, J., & Wobus, A. M. (2001). Embryonic stem cell differentiation: the role of extracellular factors. Differentiation, 68(4–5), 167–174.
Chen, L., Mizutani, A., Kasai, T., et al. (2014). Mouse induced pluripotent stem cell microenvironment generates epithelial-mesenchymal transition in mouse lewis lung cancer cells. American Journal of Cancer Research, 4(1), 80–88.
Bissell, M. J., & Barcellos-Hoff, M. H. (1987). The influence of extracellular matrix on gene expression: is structure the message? Journal of Cell Science, 8, 327–343.
Gentleman, E., Swain, R. J., Evans, N. D., et al. (2009). Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nature Materials, 8(9), 763–70.
Li, Y., Liu, M., Yan, Y., & Yang, S. T. (2014). Neural differentiation from pluripotent stem cells: the role of natural and synthetic extracellular matrix. World Journal of Stem Cells, 6(1), 11–23.
Turner, D. A., Trott, J., Hayward, P., Rue, P., & Arias, A. M. (2014). An interplay between extracellular signaling and the dynamics of the exit from pluripotency drives cell fate decisions in mouse ES cells. Biology Open, 3(7), 614–26.
Wilschut, K. J., Haagsmanb, H. P., & Roelena, B. A. J. (2010). Extracellular matrix components direct porcine muscle stem cell behavior. Experimental Cell Research, 316(3), 341–352.
Liu, Z., Scannell, D. R., Eisen, M. B., & Tjian, R. (2011). Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell, 146(5), 720–731.
Irwin, E., Gupta, R., Dashti, D., & Healy, K. E. (2011). Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials, 32(29), 6912–6919.
Cui, Y., Lu, C., Meng, D., et al. (2014). Collagen scaffolds modified with CNTF and BFGF promote facial nerve regeneration in minipigs. Biomaterials, 35(27), 7819–7827.
Giancotti, F. G., & Ruoslahti, E. (1999). Integrin signaling. Science, 285(5430), 1028–1032.
Li, L., Sun, L., Gao, F., et al. (2010). Stk40 links the pluripotency factor Oct4 to the Erk/MAPK pathway and controls extra-embryonic endoderm differentiation. Proceedings of the National Academy of Sciences of the United States of America, 107(4), 1402–1407.
Dzobo, K., Leaner, V. D., & Parker, M. I. (2012). Feedback regulation of the α2(1) collagen gene via the Mek–Erk signaling pathway. IUBMB Life, 64(1), 87–98.
Chen, H., Chen, X., & Zheng, Y. (2013). The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling. Cell Stem Cell, 13(1), 73–86.
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P., & Brivanlou, A. H. (2004). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine, 10(1), 55–63.
Bikkavilli, R. K., & Malbon, C. C. (2009). Mitogen-activated protein kinases and Wnt/B-catenin signaling. Molecular conversations among signaling pathways. Communicative and Integrative Biology, 2(1), 46–49.
Nakanishi, M., Kurisaki, A., Hayashi, Y., et al. (2009). Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. The FASEB Journal, 23(1), 114–122.
Bone, H. K., Nelson, A. S., Goldring, C. E., Tosh, D., & Welham, M. J. (2011). A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. Journal of Cell Science, 124(12), 1992–2000.
Serebriiskii, I., Castelló-Cros, R., Lamb, A., Golemis, E. A., & Cukierman, E. (2008). Fibroblast-derived 3D matrix differentially regulates the growth and drug- responsiveness of human cancer cells. Matrix Biology, 27(6), 573–585.
Dzobo, K., Leaner, V. D., & Parker, M. I. (2014). Absence of feedback regulation in the synthesis of COL1A1. Life Sciences, 103(1), 25–33.
Jang, M., Lee, S. T., Kim, J. W., et al. (2014). Excitation propagation in three-dimensional engineered hearts using decellularised extracellular matrix. Biomaterials, 35, 7839–7850.
Yoon, B. S., Yoo, S. J., Lee, J. E., You, S., Lee, H. T., & Yoon, H. S. (2006). Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation, 74(4), 149–159.
Chomczynski, P., & Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry, 162(1), 156–159.
Crews, C. M., Alessandrini, A., & Erikson, R. L. (1992). The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science, 258(5081), 478–80.
Aparicio, I. M., Garcia-Herreros, M., Fair, T., & Lonergan, P. (2010). Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction, 140, 83–92.
Damien, C. J., & Parsons, J. R. (1991). Bone graft and bone graft substitutes: a review of current technology and applications. Journal of Applied Biomaterials, 2(3), 187–208.
Pera, M. F., & Tam, P. P. L. (2010). Extrinsic regulation of pluripotent stem cells. Nature Review Insight, 465(7299), 713–720.
Haque, M. A., Nagaoka, M., Hexing, B., & Akaike, T. (2010). Artificial extracellular matrix for embryonic stem cell cultures: a new frontier of nanobiomaterials. Science and Technology of Advanced Materials, 11, 014106 (9 pp).
Wei, J., Han, J., Zhao, Y., et al. (2014). The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials, 35(27), 7724–7733.
Schenke-Layland, K., Angelis, E., Rhodes, K. E., Heydarkhan-Hagvall, S., Mikkola, H. K., & Maclellan, W. R. (2007). Collagen IV induces trophoectoderm differentiation of mouse embryonic stem cells. Stem Cells, 25(6), 1529–38.
Coraux, C., Hilmi, C., Rouleau, M., et al. (2003). Reconstituted skin from murine embryonic stem cells. Current Biology, 13(10), 849–53.
Bandi, S., Cheng, K., Joseph, B., & Gupta, S. (2012). Spontaneous origin from human embryonic stem cells of early developmental stage liver cells displaying conjoint meso-endodermal phenotype with hepatic functions. Journal of Cell Science, 125(5), 1274–83.
Greenlee, A. R., Kronenwetter-Koepel, T. A., Kaiser, S. J., & Liu, K. (2005). Comparison of Matrigel and gelatin substrata for feeder-free culture of undifferentiated mouse embryonic stem cells for toxicity testing. Toxicology in Vitro, 19(3), 389–397.
Palklin, S., & Vallier, L. (2013). The cell-cycle state of stem cells determines cell fate propensity. Cell, 155(1), 135–147.
Zhou, X., Murphy, F. R., Gehdu, N., Zhang, J., Iredale, J. P., & Benyon, R. C. (2004). Engagement of αvβ3 integrin regulates proliferation and apoptosis of hepatic stellate cells. The Journal of Biological Chemistry, 279(23), 23996–24006.
Huang, M. L., Smith, R. A. A., Trieger, G. W., & Godula, K. (2014). Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. Journal of the American Chemical Society, 136(30), 10565–10568.
Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A., & Meur, M. L. (1996). Absence of integrin 6 leads to epidermolysis bullosa and neonatal death in mice. Nature Genetics, 13(3), 370–373.
Welser, J. V., Rooney, J. E., Cohen, N. C., et al. (2009). Myotendinous junction defects and reduced force transmission in mice that lack α7 integrin and utrophin. The American Journal of Pathology, 175(4), 1545–1554.
Hynes, R. O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell, 69, 11–25.
Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell, 110(6), 673–687.
Giancotti, F. G. (2003). A structural view of integrin activation and signaling. Developmental Cell, 4(2), 149–151.
Brown, E. J. (2002). Integrin-associated proteins. Current Opinion in Cell Biology, 14(5), 603–607.
Bakre, M. M., Hoi, A., Mong, J. C. Y., Koh, Y. Y., Wong, K. Y., & Stanton, L. W. (2007). Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. Journal of Biological Sciences, 282(43), 31703–31712.
Davidson, K. C., Adams, A. M., Goodson, J. M., et al. (2012). Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proceedings of the National Academy of Sciences of the United States of America, 109(12), 4485–4490.
Georgopolous, N. T., Kirkwood, L. A., & Southgate, J. (2014). A novel bidirectional positive-feedback loop between Wnt-B-catenin and EGFR-ERK plays a role in context-specific modulation of epithelial tissue regeneration. Journal of Cell Science, 127(13), 2967–2982.
Grossmann, T. N., Yeh, J. T., Bowman, B. R., Chu, Q., Moellering, R. E., & Verdine, G. L. (2012). Inhibiting the oncogenic Wnt signaling through direct targeting of β-catenin. Proceedings of the National Academy of Sciences of the United States of America, 109(44), 17942–17947.
Li, J., Wang, G., Wang, C., et al. (2007). MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation, 75(4), 299–307.
Burdon, T., Smith, A., & Savatier, P. (2002). Signalling, cell cycle and pluripotency in embryonic stem cells. Trends in Cell Biology, 12(9), 432–438.
Acknowledgments
This work was supported by the International Centre for Genetic Engineering and Biotechnology (ICGEB), the South African Medical Research Council and the University of Cape Town. The funders had no role in the conduct of the research or the preparation of the manuscript.
Conflicts of Interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig S1
Densitometric quantification of western blot gels, showing stem cell markers, Oct4, Sox2 and Ssea4 downregulation in ESCs cultured on fd-ECM a After 4 days of incubation b After 8 days of incubation c After 16 days of incubation d After 24 days of incubation. Data are presented as mean ± standard deviation (GIF 308 kb)
Supplemental Fig S2
a Experimental design of induced hESCs differentiation through the hanging drop method. b A representative image of hESCs BG01V cultured on feeder layer of MEFs c A representative image of the hanging drop method (GIF 304 kb)
Supplemental Fig S3
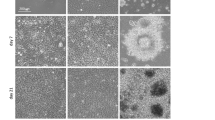
Effect of different ECM coatings on hESCs differentiation a hESCs BG01V cultured on feeder layer of MEFs then differentiated by the hanging drop (HD) method and suspension culture. Images are for embryoid bodies day 2,4 and 8 b hESCs BG01V cultured on fd-ECM and treated as in (A). Images are for embryoid bodies day 2,4 and 8 c hESCs BG01V cultured on Matrigel and treated as in (A). Images are for embryoid bodies day 2,4 and 8 d hESCs BG01V cultured on collagen and treated as in (a). Images are for embryoid bodies day 2,4 and 8. Scale bar: 25 μm (GIF 359 kb)
Supplemental Fig S4
a Western blot analysis of embryoid bodies samples after incubation for the indicated days using Oct4, Sox-2 and Ssea-4 antibodies. The levels of β-tubulin in the same sample were used as a loading control. (b to c) RT PCR analysis of OCT4, NANOG, AXIN2 and LEF1 expression in embryoid bodies from different ECM coatings. GAPDH was used as the normalizer. d Detection of integrin-binding sites on the fd-ECM. Integrin receptors were blocked and attached cells were quantified through the use of crystal violet staining. Quantification was done relative to cells that were not incubated with blocking antibodies which was taken as 100 % attachment. Data are presented as mean ± standard deviation, * p < 0.05 (GIF 220 kb)
Supplemental Table S1
Molecular weights of proteins analyzed by western blot (DOC 76 kb)
Supplemental Table S2
Oligonucleotide primer sequences used for real time quantitative PCR (DOC 258 kb)
Rights and permissions
About this article
Cite this article
Dzobo, K., Vogelsang, M. & Parker, M.I. Wnt/β-Catenin and MEK-ERK Signaling are Required for Fibroblast-Derived Extracellular Matrix-Mediated Endoderm Differentiation of Embryonic Stem Cells. Stem Cell Rev and Rep 11, 761–773 (2015). https://doi.org/10.1007/s12015-015-9598-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9598-4