Abstract
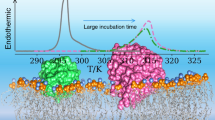
Combined effects of flunitrazepam (FNZ) and lidocaine (LDC) were studied on the thermotropic equilibrium of dipalmitoyl phosphatidylcholine (dpPC) bilayers. This adds a thermodynamic dimension to previously reported geometric analysis in the erythrocyte model. LDC decreased the enthalpy and temperature for dpPC pre- and main-transitions (ΔH p, ΔH m, T p, T m) and decreased the cooperativity of the main-transition (ΔT 1/2,m). FNZ decreased ΔH m and, at least up to 59 μM, also decreased ΔH p. In conjunction with LDC, FNZ induced a recovery of ∆T 1/2,m control values and increased ΔH m even above the control level. The deconvolution of the main-transition peak at high LDC concentrations revealed three components possibly represented by: a self-segregated fraction of pure dpPC, a dpPC–LDC mixture and a phase with a lipid structure of intermediate stability associated with LDC self-aggregation within the lipid phase. Some LDC effects on thermodynamic parameters were reverted at proper LDC/FNZ molar ratios, suggesting that FNZ restricts the maximal availability of the LDC partitioned into the lipid phase. Thus, beyond its complexity, the lipid–LDC mixture can be rationalized as an equilibrium of coexisting phases which gains homogeneity in the presence of FNZ. This work stresses the relevance of nonspecific drug–membrane binding on LDC–FNZ pharmacological interactions and would have pharmaceutical applications in liposomal multidrug-delivery.








Similar content being viewed by others
Abbreviations
- BZD:
-
Benzodiazepine
- dpPC:
-
Dipalmitoyl phosphatidylcholine
- DSC:
-
Differential scanning calorimetry
- FNZ:
-
Flunitrazepam
- LDC:
-
Lidocaine
- MLVs:
-
Multilamellar vesicles
References
Sloan, J. W., Martin, W. R., & Wala, E. P. (1991). A comparison of the physical dependence inducing properties of flunitrazepam and diazepam. Pharmacology, Biochemistry and Behavior, 39(2), 395–405.
Smith, C. (1994). Pharmacology of local anaesthetic agents. British Journal of Hospital Medicine, 52(9), 455–460.
Rudolph, U., & Möhler, H. (2004). Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annual Review of Pharmacology and Toxicology, 44, 475–498.
McLure, H. A., & Rubin, A. P. (2005). Review of local anaesthetic agents. Minerva Anestesiologica, 71, 59–74.
Biswas, S., et al. (1999). Low-dose midazolam infusion for oculoplastic surgery under local anesthesia. Eye (Lond), 13(Pt 4), 537–540.
Deng, X., et al. (2001). The use of midazolam and small-dose ketamine for sedation and analgesia during local anesthesia. Anesthesia and Analgesia, 93(5), 1174–1177.
Cinnella, G., et al. (2007). Sedation analgesia during office-based plastic surgery procedures: Comparison of two opioid regimens. Plastic and Reconstructive Surgery, 119(7), 2263–2270.
Badrinath, S., et al. (2000). The use of a ketamine-propofol combination during monitored anesthesia care. Anesthesia and Analgesia, 90(4), 858–862.
Neal, J. M., et al. (2010). ASRA practice advisory on local anesthetic systemic toxicity. Regional Anesthesia and Pain Medicine, 35(2), 152–161.
Rudolph, H., et al. (1981). Benzodiazepines protect mice from local anesthetic convulsions and deaths. Anesthesia and Analgesia, 60(6), 385–389.
Hogan, Q. (1996). Local anesthetic toxicity: An update. Regional Anesthesia, 21(6), 43–50.
Weinberg, G. L. (2010). Treatment of Local Anesthetic Systemic Toxicity (LAST). Regional Anesthesia and Pain Medicine, 35(2), 188–193.
Gavish, M., et al. (1992). Biochemical, physiological, and pathological aspects of the peripheral benzodiazepine receptor. Journal of Neurochemistry, 58(5), 1589–1601.
Stephenson, F. A. (1998). Understanding the GABAA receptor: A chemically gated ion channel. Biochememical Journal, 249, 21–32.
Weinkauf, B., et al. (2012). Differential effects of lidocaine on nerve growth factor (NGF)-evoked heat- and mechanical hyperalgesia in humans. European Journal of Pain, 16(4), 543–549.
Perillo, M. A., & Arce, A. (1991). Determination of the membrane-buffer partition coefficient of flunitrazepam, a lipophilic drug. Journal of Neuroscience Methods, 36(2–3), 203–208.
Perillo, M., Garcia, D. A., & Arce, A. (1995). Partitioning of 1,4 benzodiazepines into natural membranes. Molecular Membrane Biology, 11, 217–224.
de Paula, E., & Schreier, S. (1995). Use of a novel method for determination of partition coefficients to compare the effect of local anesthetics on membrane structure. Biochimica et Biophysica Acta, 1240, 25–33.
Fernandes Fraceto, L., et al. (2002). Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophysical Chemistry, 99(3), 229–243.
de Paula, E., et al. (2008). Preferential location of lidocaine and etidocaine in lecithin bilayers as determined by EPR, fluorescence and 2H NMR. Biophysical Chemistry, 132(1), 47–54.
Seelig, A., Allegrini, P. R., & Seelig, J. (1988). Partitioning of local anesthetics into membranes: Surface charge effects monitored by the phospholipid head-group. Biochimica et Biophysica Acta, 939(2), 267–276.
García, D. A., & Perillo, M. A. (1997). Localization of flunitrazepam in artificial membranes. A spectrophotometric study about the effect the polarity of the medium exerts on flunitrazepam acid-base equilibrium. Biochimica et Biophysica Acta, 1324(1), 76–84.
García, D. A., & Perillo, M. A. (1997). Partitioning of flunitrazepam into model membranes studied by temperature controlled gel filtration chromatography. Biomedical Chromatography, 11(6), 343–347.
García, D. A., & Perillo, M. A. (1999). Benzodiazepine localisation at the lipid–water interface: Effect of membrane composition and drug chemical structure. Biochimica et Biophysica Acta, 1418(1), 221–231.
Perillo, M. A., & Garcia, D. A. (2001). Flunitrazepam induces geometrical changes at the lipid–water interface. Colloids and Surfaces B: Biointerfaces, 20(1), 63–72.
García, D. A., Quiroga, S., & Perillo, M. A. (2000). Flunitrazepam partitioning into natural membranes increases surface curvature and alters cellular morphology. Chemico-Biological Interactions, 129(3), 263–277.
Chen, J. Y., & Huestis, W. H. (1997). Role of membrane lipid distribution in chlorpromazine-induced shape change of human erythrocytes. Biochimica et Biophysica Acta, 1323(2), 299–309.
Cullis, P. R., et al. (1997). Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochimica et Biophysica Acta, 1331(2), 187–211.
Rasia, M., & Bollini, A. (1998). Red blood cell shape as a function of medium’s ionic strength and pH. Biochimica et Biophysica Acta, 1372(2), 198–204.
Petrov, P. G., & Döbereiner, H. G. (2000). Light-induced shape transitions of giant vesicles. In P. L. Luisi & P. Walde (Eds.), Giant vesicles (pp. 336–339). Chichester: Wiley.
Nishiguchi, E., Sindo, J., & Hamasaki, N. (1993). Requirement of cytoplasmic components for lidocaine-induced shape change in human erythrocytes. Biochimica et Biophysica Acta, 1176(1–2), 95–105.
Huang, C.-h, & Li, S. (1999). Calorimetric and molecular mechanics studies of the thermotropic phase behavior of membrane phospholipids. Biochimica et Biophysica Acta, 1422(3), 273–307.
Hata, T., Matsuki, H., & Kaneshina, S. (2000). Effect of local anesthetics on the bilayer membrane of dipalmitoylphosphatidylcholine: Interdigitation of lipid bilayer and vesicle-micelle transition. Biophysical Chemistry, 87(1), 25–36.
Takeda, K., et al. (2009). Effect of local anaesthetic lidocaine hydrochloride on the bilayer structure of phospholipids. Journal of Oleo Science, 58, 369–377.
Matsingou, C., & Demetzos, C. (2007). Calorimetric study on the induction of interdigitated phase in hydrated DPPC bilayers by bioactive labdanes and correlation to their liposome stability: The role of chemical structure. Chemistry and Physics of Lipids, 145(1), 45–62.
Moraes, C. M. (2007). Preparação e caracterização físico-química de complexos de inclusão entre anestésicos locais e hidroxi propil-β-ciclodextrina. Química Nova, 30, 777–784.
Barceloux, D. G. (2012). Medical toxicology of drugs abuse: Synthesized chemicals and psychoactive plants. Hoboken, NJ: Wiley.
Ueda, I., et al. (1994). Local anesthetics destabilize lipid membranes by breaking hydration shell: Infrared and calorimetry studies. Biochimica et Biophysica Acta, 1190(2), 421–429.
Malheiros, S. V. P., et al. (2004). A new look at the hemolytic effect of local anesthetics, considering their real membrane/water partitioning at pH 7.4. Biophysical Chemistry, 110(3), 213–221.
Garcia, D. A., & Perillo, M. A. (1997). Supramolecular events modulate flunitrazepam partitioning into natural and model membranes. Colloids Surfaces B: Biointerfaces, 9, 49–57.
Frye, J., et al. (1985). Cross polarization P-31 nuclear magnetic resonance of phospholipids. Biophysical Journal, 48(4), 547–552.
Koynova, R., & Caffrey, M. (1998). Phases and phase transitions of the phosphatidylcholines. Biochimica et Biophysica Acta, 1376(1), 91–145.
Almeida, A. C. P.d. (2008). Lidocaína lipossomal produzida em processo escalonável: formulação, caracterização e testes biológicos. Campinas: Universidade Estadual de Campinas.
Mitragotri, S., et al. (1999). An analysis of the selectivity of solute partitioning. Diffusion and permeation across lipid bilayer. Biophysical Journal, 77, 1268–1283.
Fernandes Fraceto, L., et al. (2005). Differential effects of uncharged aminoamide local anesthetics on phospholipid bilayers, as monitored by 1H-NMR measurements. Biophysical Chemistry, 115(1), 11–18.
de Verteuil, F., et al. (1981). Phase diagrams for impure lipid systems: Application to lipid/anaesthetic mixtures. Biochimica et Biophysica Acta, 640(1), 207–222.
Hata, T., et al. (2001). Partition coefficients of charged and uncharged local anesthetics into dipalmitoylphosphatidylcholine bilayer membrane: Estimation from pH dependence on the depression of phase transition temperatures. Colloids and Surfaces B: Biointerfaces, 22(1), 77–84.
Pasenkiewicz-Gierula, M., et al. (2003). Effects of a carane derivative local anesthetic on a phospholipid bilayer studied by molecular dynamics simulation. Biophysical Journal, 85(2), 1248–1258.
Lorite, G. S., et al. (2009). Dibucaine effects on structural and elastic properties of lipid bilayers. Biophysical Chemistry, 139, 75–83.
Fernandez, M. F. (1981). Disruption of liposomes by tetracaine micelles. Biochimica et Biophysica Acta, 647, 27–30.
Hamill, O. P., & Martinac, B. (2001). Molecular basis for mechanotransduction in living cells. Physiological Reviews, 81, 685–740.
Inoko, Y., & Mitsui, T. (1978). Structural parameters of dipalmitoyl phosphatidylcholine lamellar phases and bilayer phase transitions. Journal of Physiological Society of Japan, 44, 1918–1924.
Kodama, M., Kuwabara, M., & Seki, S. (1982). Successive phase-transition phenomena and phase diagram of the phosphatidylcholine–water system as revealed by differential scanning calorimetry. Biochimica et Biophysica Acta, 689(3), 567–570.
Wildsmith, J. A. W., et al. (1987). Differential nerve-blockade: Esters vs. amides and the influence of pKa. British Journal of Anaesthetics, 59, 379–384.
Fujii, T., et al. (1979). Shape changes of human erythrocytes induced by various amphipathic drugs acting on the membrane of the intact cells. Biochemical Pharmacology, 28, 613–620.
Mattila, M. A., et al. (1983). The efficacy and plasma concentrations of flunitrazepam after oral or intramuscular premedication. International Journal of Clinical, Pharmacology, Therapeutics and Toxicology, 21, 284–286.
Grahnen, A., et al. (1991). Inter- and intraindividual variability in the concentration-effect (sedation) relationship of flunitrazepam. British Journal of Clinical Pharmacology, 31, 89–92.
de Paula, E., et al. (2012). Micro and nanosystems for delivering local anesthetics. Expert Opinion on Drug Delivery, 9, 1505–1524.
Rousseau, G. F., et al. (2002). Plasma lidocaine concentrations following insertion of 2% lidocaine gel into the uterine cavity after uterine balloon thermal ablation. British Journal of Anaesthetics, 89, 846–848.
Atanassoff, P. G., Weiss, B. M., & Brull, S. J. (1996). Lidocaine plasma levels following two techniques of obturator nerve block. Journal of Clinical Anesthesiology, 8, 535–539.
Merle, J. C., et al. (1999). A comparison of two techniques for cervical plexus blockade: Evaluation of efficacy and systemic toxicity. Anesthesia and Analgesia, 89, 1366–1370.
Acknowledgments
This work was partially financed with grants from a bilateral CAPES (Brasil)/SPU(Argentina) project (# Project CAPG-BA 06/02), Fapesp (# 06/00121-9), SeCyT-UNC, Mincyt-Córdoba and CONICET (Argentina). JMS, DAG, and MAP are career investigators from CONICET and BC holds a postdoctoral fellowship from the later institution. EP has a fellowship from CNPq/Brazil. Authors gratefully acknowledge Dr M. L. Bianconi from UFRJ (Brazil) for the help with DSC experiments at the early stages of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Benjamín Caruso and Julieta M. Sánchez contributed equally to this work.
Rights and permissions
About this article
Cite this article
Caruso, B., Sánchez, J.M., García, D.A. et al. Probing the Combined Effect of Flunitrazepam and Lidocaine on the Stability and Organization of Bilayer Lipid Membranes. A Differential Scanning Calorimetry and Dynamic Light Scattering Study. Cell Biochem Biophys 66, 461–475 (2013). https://doi.org/10.1007/s12013-012-9494-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9494-3