Abstract
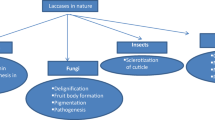
Lignin is amorphous in nature, lacks stereoregularity, and is not susceptible to hydrolytic attack. Despite its resistant nature, it is however degraded by various microorganisms, particularly, white-rot fungi. Such fungi are capable of extracellular production of lignin peroxidase, manganese peroxidase, and laccase, the three major enzymes associated with ligninolysis. Though all white-rot fungi do not produce all the three enzymes, laccase occupies an important place in ligninolysis. Laccase belongs to a diverse group of enzymes called oxidoreductases and is also known as benzenediol: oxygen oxidoreductase. They have low substrate specificity. The copper-containing enzyme laccase has been detected in a variety of organisms such as bacteria, fungi, plants, and insects. Mostly, these are extracellular proteins, although intracellular laccases have also been detected in some fungi and insects. Fungal laccases are believed to play a variety of roles, such as, morphogenesis, pathogenesis, and lignin degradation. As an oxidase, laccase is used in many agricultural, industrial, and medicinal applications. Current investigations are focused on laccase-based biooxidation, biotransformation, biosensor, and enzymatic synthesis of organic compounds. By enhancing laccase production using different physiochemical parameters, better understanding of the mechanism for the reactions of interest, and optimizing the catalytic activity of laccase, it can be used in a better way in diverse fields of biotechnology.






Similar content being viewed by others
References
Dean, J. F. D., & Eriksson, K. E. (1992). Biotechnological modification of lignin structure and composition in forest trees. Holzforschung, 46, 135–147.
Alder, E. (1977). Lignin chemistry, past, present and future. Wood Science and Technology, 11, 169–218. doi:10.1007/BF00365615.
Eriksson, K. E., Grunewald, A., & Vallander, L. (1980). Studies on growth conditions in wood for three white rot fungi and their cellulase-less mutants. Biotechnology and Bioengineering, 22, 363–376. doi:10.1002/bit.260220210.
Råberg, U., Terziev, N., & Land, C. J. (2009). Early soft rot colonization of Scots sapwood pine in above-ground exposure. International Biodeterioration & Biodegradation, 63, 236–240. doi:10.1016/j.ibiod.2007.10.005.
Rabinovich, M. L., Bolobova, A. V., & Vasil'chenko, L. G. (2004). Fungal decomposition of natural aromatic structures and xenobiotics: A review. Applied Biochemistry and Microbiology, 40, 1–17. doi:10.1023/B:ABIM.0000010343.73266.08.
Nilsson, T., Daniel, G., Kirkk, T. K., & Obst, J. R. (1989). Chemistry and microscopy of wood decay by some higher ascomycetes. Holzforschung, 43, 11–18.
Blanchette, R. A., Held, B. W., Jurgens, J. A., McNew, D. L., Harrington, T. C., Duncan, S. M., et al. (2004). Wood destroying soft rot fungi in the historic expedition huts of antarctica. Applied and Environmental Microbiology, 70, 1328–1335. doi:10.1128/AEM.70.3.1328-1335.2004.
Highley, T. L., & Illman, B. L. (1991). Progress in understanding how brown-rot fungi degrade cellulose. Biodeterioration Abstracts, 5, 231–244.
Kirk, T. K. (1975). Chemistry of lignin degradation by wood-destroying fungi. In W. Liese (Ed.), Biological transformation of wood by microorganisms. Berlin: Springer.
Jin, L., Nicholas, D. D., & Kirk, T. K. (1990). Mineralization of the methoxy carbon of isolated lignin by brown rot fungi under solid substrate conditions. Wood Science and Technology, 24, 263–276. doi:10.1007/BF01153559.
Boyley, C. D., Kropp, B. R., & Reid, I. D. (1992). Solubilization and mineralization of lignin by white rot fungi. Applied and Environmental Microbiology, 58, 3217–3224.
Hatakka, A. (1994). Lignin-modifying enzymes from selected white rot fungi: Production and role in lignin degradation. FEMS Microbiology Reviews, 13, 125–135. doi:10.1111/j.1574-6976.1994.tb00039.x.
Nerud, F., & Misurcova, Z. (1996). Distribution of ligninolytic enzymes in selected white rot fungi. Folia Microbiologica, 41, 264–266. doi:10.1007/BF02814628.
Riva, S. (2006). Laccases: Blue enzymes for green chemistry. Trends in Biotechnology, 5, 219–225. doi:10.1016/j.tibtech.2006.03.006.
Baldrian, P. (2004). Purification and characterization of laccase from the white rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Applied Microbiology and Biotechnology, 63, 560–563. doi:10.1007/s00253-003-1434-0.
Harkin, J. M., Larsen, M. J., & Obst, J. R. (1974). Use of syringaldazine for detection of laccase in sporophores of wood rotting fungi. Mycologia, 66, 469–476. doi:10.2307/3758490.
Ferraroni, M., Duchi, I., Myasoedova, N. M., Leontievsky, A. A., Golovleva, L. A., Scozzafava, A., et al. (2005). Crystallization and preliminary structure analysis of the blue laccase from the ligninolytic fungus Panus tigrinus. Acta Crystallographica, F61, 205–207.
Morozova, O. V., Shumakovich, G. P., Gorbacheva, M. A., Shleev, S. V., & Yaropolov, A. I. (2007). Blue. Laccases. Biochemistry (Moscow), 72, 1136–1150. doi:10.1134/S0006297907100112.
Pozdnyakova, N. N., Turkovskaya, O. V., Yudina, E. N., & Rodakiewicz-Nowak, Y. (2006). Yellow laccase from the fungus Pleurotus ostreatus D1. Purification and characterization. Applied Biochemistry and Microbiology, 42, 56–61.
Leontievsky, A. A., Vares, T., Lankinen, P., Shergill, J. K., Pozdnyakova, N. N., Myasoedova, N. M., et al. (1997). Blue and yellow laccases of ligninolytic fungi. FEMS Microbiology Letters, 156, 9–14.
Palmieri, G., Cennamo, G., Faraco, V., Amoresano, A., Sannia, G., & Giardina, P. (2003). Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme and Microbial Technology, 33, 220–230. doi:10.1016/S0141-0229(03)00117-0.
Jordaan, J., Pletschke, B. I., & Leukes, W. D. (2004). Purification and partial characterization of a thermostable laccase from an unidentified basidiomycete. Enzyme and Microbial Technology, 34, 635–641. doi:10.1016/j.enzmictec.2004.02.003.
Ranocha, P., Chabannes, M., Chamayou, S., Danoun, S., Jauneau, A., Boudet, A. M., et al. (2002). Laccase down-regulation causes alteration in phenolic metabolism and cell wall structure in poplar. Plant Physiology, 129, 1–11. doi:10.1104/pp.010988.
De’Souza, T. M., Boomminathan, K., & Reddy, C. A. (1996). Isolation of laccase gene-specific sequences from white rot and brown rot fungi by PCR. Applied and Environmental Microbiology, 62, 3739–3744.
Palaez, F., Martínez, M. J., & Martínez, A. T. (1995). Screening of 68 species of basidiomycetes involved in lignin degradation. Mycological Research, 99, 37–42. doi:10.1016/S0953-7562(09)80313-4.
Ruttimann, C., Schwember, E., Salas, L., Cullen, D., & Vicuna, R. (1992). Ligninolytic enzymes of the white-rot basidiomycetes Phlebia brevispora and Ceriporiopsis subvermispora. Biotechnology and Applied Biochemistry, 16, 64–76.
Jimenez, M., Gonzalez, A. E., Martinez, M. J., Martinez, A. T., & Dale, B. E. (1991). Screening of yeasts isolated from decayed wood for lignocellulose degrading enzyme activities. Mycological Research, 95, 1299–1302. doi:10.1016/S0953-7562(09)80578-9.
Williamson, P. R. (1994). Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. Journal of Bacteriology, 176, 656–664.
Svobodova, K. (2005). The implication of ligninolytic enzymes in the decolorization of synthetic dyes by white rot fungus Irpex lacteus. Ph.D. Thesis, Charles University, Prague, Czech Republic.
Zhu, X. D., Gibbons, J., Garcia-Rivera, J., Casadevall, A., & Williamson, P. R. (2001). Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infection and Immunity, 69, 5589–5596. doi:10.1128/IAI.69.9.5589-5596.2001.
Holker, U., Dohse, J., & Hofer, M. (2002). Extracellular laccase in ascomycetes Trichoderma atroviride and Trichoderma harzianum. Folia Microbiologica, 47, 423–427. doi:10.1007/BF02818702.
Cairney, J. W. D., & Burke, R. M. (1998). Do ecto- and ericoid mycorrhizal fungi produce peroxidase activity? Mycorrhiza, 8, 61–65. doi:10.1007/s005720050213.
Burke, R. M., & Cairney, J. W. G. (2002). Laccase and other polyphenol oxidases in ecto- and ericoid mycorrhizal fungi. Mycorrhiza, 12, 105–116.
Chen, D. M., Bastias, B. A., Taylor, A. F. S., & Cairney, J. W. G. (2003). Identification of laccase-like genes in ectomycorrhizal basidiomycetes and transcriptional regulation by nitrogen in Piloderma byssinum. The New Phytologist, 157, 547–554. doi:10.1046/j.1469-8137.2003.00687.x.
Luis, P., Walther, G., Kellner, H., Martin, F., & Buscot, F. (2004). Diversity of laccase genes from basidiomycetes in a forest soil. Soil Biology & Biochemistry, 36, 1025–1036. doi:10.1016/j.soilbio.2004.02.017.
Gunther, H., Perner, B., & Grammes, G. (1998). Activities of phenol oxidizing enzymes of ectomycorrhizal fungi in axenic culture and in symbiosis with Scots pine (Pinus sylvestris L.). Journal of Basic Microbiology, 38, 197–206. doi:10.1002/(SICI)1521-4028(199807)38:3<197::AID-JOBM197>3.0.CO;2-W.
Timonen, S., & Sen, R. (1998). Heterogeneity of fungal and plant enzyme expression in intact Scots pine Suillus bovines and Paxillus involutus mycorrhizospheres developed in natural forest humus. The New Phytologist, 138, 355–366. doi:10.1046/j.1469-8137.1998.00103.x.
Suzuki, T., Endo, K., Ito, M., Tsujibo, H., Miyamoto, K., & Inamori, Y. (2003). A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Bioscience, Biotechnology, and Biochemistry, 67, 2167–2175. doi:10.1271/bbb.67.2167.
Claus, H. (2003). Laccases and their occurrence in prokaryotes. Archives of Microbiology, 179, 145–150.
Givaudan, A., Effosse, A., Faure, D., Potier, P., Bouillant, M. L., & Bally, R. (1993). Polyphenol oxidase from Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of A. lipoferum. FEMS Microbiology Letters, 108, 205–210. doi:10.1111/j.1574-6968.1993.tb06100.x.
Alexandre, G., & Zhulin, I. B. (2000). Laccases are wide spread in bacteria. Trends in Biotechnology, 18, 41–42. doi:10.1016/S0167-7799(99)01406-7.
Enguita, F. J., Martins, L. O., Henriques, A. O., & Carrondo, M. A. (2003). Crystal structure of a bacterial endospore coat component: A laccase with enhanced thermostability properties. The Journal of Biological Chemistry, 278, 19416–19425. doi:10.1074/jbc.M301251200.
Galai, S., Limam, F., & Marzouki, M. N. (2008). A new Stenotrophomonas maltophilia strain producing laccase. Use in decolorization of synthetic dyes. Applied Biochemistry and Biotechnology, in press.
Xu, F. (1999). Laccase. In M. C. Flickinger & S. W. Drew (Eds.), The encyclopedia of bioprocessing technology: Fermentation, biocatalysis and bioseparation (pp. 1545–1554). New York: Wiley.
Bailey, M. R., Woodard, S. L., Callawy, E., Beifuss, K., Magallanes-Lundback, M., Lane, J., et al. (2004). Improved recovery of active recombinant laccase from maize seed. Applied Microbiology and Biotechnology, 63, 390–397. doi:10.1007/s00253-003-1362-z.
Dittmer, J. K., Patel, N. J., & Dhawale, S. W. (1997). Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS Microbiology Letters, 149, 65–70. doi:10.1111/j.1574-6968.1997.tb10309.x.
Nagai, M., Kawata, M., Watanabe, H., Ogawa, M., Saito, K., Takesawa, T., et al. (2003). Important role of fungal intracellular laccase for melanin synthesis: Purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology, 149, 2455–2462. doi:10.1099/mic.0.26414-0.
Min, K. L., Kim, Y. H., Kim, Y. W., Jung, H. S., & Hah, Y. C. (2001). Characterization of a novel laccase produced by the wood-rotting fungus Phellinus ribis. Archives of Biochemistry and Biophysics, 392, 279–286. doi:10.1006/abbi.2001.2459.
De Souza, C. G. M., & Peralta, R. M. (2003). Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. Journal of Basic Microbiology, 43, 278–286. doi:10.1002/jobm.200390031.
Yaver, D. S., Xu, F., Golightly, E. J., Brown, K. M., Brown, S. H., Rey, M. W., et al. (1996). Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Applied and Environmental Microbiology, 62, 834–841.
Palmieri, G., Giardina, P., Bianco, C., Scaloni, A., Capassoi, A., & Sannia, G. (1997). A novel white laccase from Pleurotus ostreatus. The Journal of Biological Chemistry, 272, 31301–31307. doi:10.1074/jbc.272.50.31301.
Thurston, C. F. (1994). The structure and function of fungal laccases. Microbiology, 140, 19–26.
Ng, T. B., & Wang, H. X. (2004). A homodimeric laccase with unique characteristics from the yellow mushroom Cantharellus cibarius. Biochemical and Biophysical Research Communications, 313, 37–41. doi:10.1016/j.bbrc.2003.11.087.
Shleev, S. V., Khan Ir, G., Morozova, O. V., Mazhugo, Y. M., Khalunina, A. S., & Yaropolov, A. I. (2004). Phenylpyrazolones, novel oxidoreductase redox mediators for degradation of xenobiotics. Applied Biochemistry and Microbiology, 40, 140–145. doi:10.1023/B:ABIM.0000018916.69491.be.
Kersten, P. J., Kalyanarman, B., Hammel, K. E., & Reinhammer, B. (1990). Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzene. The Biochemical Journal, 268, 475–480.
Higuchi, T. (1990). Lignin biochemistry: Biosynthesis and biodegradation. Wood Science and Technology, 24, 23–63. doi:10.1007/BF00225306.
Kirk, T. K., Schultz, E., Connors, W. J., Lorenz, L. F., & Zeikus, J. G. (1978). Influence of culture parameters on lignin metabolism on Phanerochaete chrysosporium. Archives of Microbiology, 117, 277–285. doi:10.1007/BF00738547.
Milstein, O., Huttermann, A., Frund, R., & Lüdemann, H. D. (1994). Enzymatic copolymerization of lignin with low-molecular mass compounds. Applied Microbiology and Biotechnology, 40, 760–767. doi:10.1007/BF00173342.
Xu, F. (1996). Oxidation of phenols, anilines and benzene thiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry, 35, 7608–7614. doi:10.1021/bi952971a.
Blaich, R., & Esser, K. (1975). Function of enzymes in wood destroying fungi. 2. Multiple forms of laccase in white rot fungi. Archives of Microbiology, 103, 271–277. doi:10.1007/BF00436360.
Kandelbauer, A., Maute, O., Kessler, R. W., Erlacher, A., & Gübitz, G. M. (2004). Study of dye decolorization in an immobilized laccase enzyme-reactor using online spectroscopy. Biotechnology and Bioengineering, 87, 552–563. doi:10.1002/bit.20162.
Xu, F., Li, K., & Elder, T. J. (2002). N-Hydroxy mediated laccase biocatalysis: Recent progress on its mechanism and future prospects of its application. Progress in Biotechnology, 21, 89–104. doi:10.1016/S0921-0423(02)80011-9.
Ferraroni, M., Myasoedova, N. M., Schmatchenko, V., Leontievsky, A. A., Golovleva, L. A., Scozzafava, A., et al. (2007). Crystal structure of a blue laccase from Lentinus tigrinus: Evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Structural Biology, 7, 60. doi:10.1186/1472-6807-7-60.
Wong, D. W. S. (2008). Structure and action mechanism of ligninolytic enzymes. Applied Biochemistry and Biotechnology, 157, 174–209. doi:10.1007/s12010-008-8279-z.
Palmieri, G., Giardina, P., Bianco, C., Fontanella, B., & Sannia, G. (2000). Copper induction of laccase isoenzymes in ligninolytic fungus Pleurotus ostreatus. Applied and Environmental Microbiology, 66, 920–924. doi:10.1128/AEM.66.3.920-924.2000.
Martinez, D., Larrondo, L. F., Putnam, N., Gelpke, M. D. S., Huang, K., Chapman, J., et al. (2004). Genome sequence of lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nature Biotechnology, 22, 695–700. doi:10.1038/nbt967.
Larrondo, L. F., Salas, L., Melo, F., Vicuña, R., & Cullen, D. (2003). A novel extracellular multicopper oxidase from Phanerochaete chrysosporium with ferroxidase activity. Applied and Environmental Microbiology, 69, 6257–6263. doi:10.1128/AEM.69.10.6257-6263.2003.
Dedeyan, B., Klonowska, A., & Tagger, S. (2000). Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Applied and Environmental Microbiology, 66, 925–929. doi:10.1128/AEM.66.3.925-929.2000.
Dion, W. M. (1951). Production and properties of a polyphenol oxidase from the fungus Polyporus versicolor. Canadian Journal of Botany, 30, 9–20. doi:10.1139/b52-002.
Westermark, U., & Eriksson, K. E. (1974). Carbohydrate-dependent enzymic quinone reduction during lignin degradation. Acta Chemica Scandinavica Series B: Organic Chemistry and Biochemistry, 28, 209–214.
Arora, D. S., & Sandhu, D. K. (1985). Survey of some Indian soils for laccase producing fungi and their lignin degrading ability. Proceedings of the Indiana Academy of Sciences, 94, 567–574.
Field, J. A., De Jong, E. D., Costa, G. F., & De Bont, J. A. M. (1992). Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Applied and Environmental Microbiology, 58, 2219–2226.
Kiiskinen, L. L., Ratto, M., & Kruus, K. (2004). Screening for novel laccase producing microbes. Journal of Applied Microbiology, 97, 640–645. doi:10.1111/j.1365-2672.2004.02348.x.
Hiroi, T., & Eriksson, K. E. (1976). Microbiological degradation of lignin. 1. Influence of cellulose on the degradation of lignins by the white rot fungus Pleurotus ostreatus. Svensk Papperstidning, 5, 157–161.
Guillen, F., Martinez, A. T., & Martinez, M. J. (1992). Substrates specificity and properties of the aryl alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. European Journal of Biochemistry, 209, 603–611. doi:10.1111/j.1432-1033.1992.tb17326.x.
Childs, R. E., & Bardsley, W. G. (1975). The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulphonic acid) as chromogen. The Biochemical Journal, 145, 93–103.
Eggert, C., Temp, U., & Eriksson, K. E. L. (1996). The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: Purification and characterization of laccase. Applied and Environmental Microbiology, 62, 1151–1158.
Geng, X., Li, K., & Xu, F. (2004). Investigation of hydroxamic acids as laccase-mediators for pulp bleaching. Applied Microbiology and Biotechnology, 64, 493–496. doi:10.1007/s00253-003-1475-4.
Ryan, S., Schnitzhofer, W., & Tranov, T. (2003). An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolorization. Enzyme and Microbial Technology, 33, 766–774. doi:10.1016/S0141-0229(03)00162-5.
Paszczynski, A., Huynk, V. B., & Crawford, R. L. (1985). Enzymatic activities of an extracellular manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiology Letters, 29, 37–41. doi:10.1111/j.1574-6968.1985.tb00831.x.
Rodriguez, A., Falcon, M. A., Carnicero, A., Perestlo, F., Fuent, G. D., & Trojanowski, J. (1996). Laccase activities of Penicillium chrysogenum in relation to lignin degradation. Applied Microbiology and Biotechnology, 45, 399–403. doi:10.1007/s002530050702.
Saparrat, M. C. N., Guillen, F., Arambarri, A. M., Martínez, A. T., & Martínez, M. J. (2002). Induction, isolation, and characterization of two laccases from the white-rot basidiomycete Coriolopsis rigida. Applied and Environmental Microbiology, 68, 1534–1540. doi:10.1128/AEM.68.4.1534-1540.2002.
Harkin, J., & Obst, J. R. (1973). Syringaldazine an effective reagent for detecting laccase and peroxidase in fungi. Experientia, 29, 381–508. doi:10.1007/BF01926734.
Galliano, H., Gas, G., & Boudet, A. M. (1990). Lignin biodegradation by cultures of Rigidoporus lignosus in solid state conditions. FEMS Microbiology Letters, 67, 295–300. doi:10.1111/j.1574-6968.1990.tb04036.x.
Ullah, M. A., Bedford, C. T., & Evans, C. S. (2000). Reactions of pentachlorophenol with laccase from Coriolus versicolor. Applied Microbiology and Biotechnology, 53, 230–234. doi:10.1007/s002530050013.
Temp, U., & Eggert, C. (1999). Novel interaction between laccase and collobiose dehydrogenase during pigment synthesis in the white rot fungi Pycnoporus cinnabarinus. Applied and Environmental Microbiology, 65, 389–395.
Arakane, Y., Muthukrishnan, S., Beeman, R. W., Kanost, M. R., & Kramer, K. J. (2005). Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proceedings of the National Academy of Sciences of the United States of America, 102, 11337–11342. doi:10.1073/pnas.0504982102.
Frases, S., Chaskes, S., Dadachova, E., & Casadevall, A. (2006). Induction by Klebsiella aerogenes of a melanin-like pigment in Cryptococcus neoformans. Applied and Environmental Biotechnology, 72, 1542–1550.
Eisenman, H. C., Mues, M., Weber, S. E., Frases, S., Chaskes, S., Gerfen, G., et al. (2007). Cryptococcus neoformans laccase catalyses melanin synthesis from both d- and l-DOPA. Microbiology, 153, 3954–3962. doi:10.1099/mic.0.2007/011049-0.
Sugareva, V., Härtl, A., Brock, M., Hübner, K., Rohde, M., Heinekamp, T., et al. (2006). Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Archives of Microbiology, 186, 345–355. doi:10.1007/s00203-006-0144-2.
Clutterbuck, A. J. (1972). Absence of laccase from yellow spored mutants of Aspergillus nidulans. Journal of General Microbiology, 70, 423–435.
Hermann, T. E., Kurtz, M. B., & Champe, S. P. (1983). Laccase localized in Hulle cells and cleistothecial primordial of Aspergillus nidulans. Journal of Bacteriology, 154, 955–964.
Leatham, G. F., & Stahmann, M. A. (1981). Studies on the laccase of Lentinula edodes: Specificity localization and association with the development of fruiting bodies. Journal of General Microbiology, 125, 147–157.
Worrell, J. J., Chet, I., & Hüttermann, A. (1986). Association of rhizomorph formation with laccase activity in Armillaria spp. Journal of General Microbiology, 132, 2527–2533.
Robene-Saustrade, I., Lung-Escarmant, B., Bonno, J. J., & Taris, B. (1992). Identification and partial characterization of an extracellular manganese-dependent phenol oxidase in Armillaria ostoyae and Armillaria mellea. European Journal of Forest Pathology, 22, 227–236. doi:10.1111/j.1439-0329.1992.tb00787.x.
Ohga, S., Cho, N. S., Thurston, C. F., & Wood, D. A. (2000). Transcriptional regulation of laccase and cellulase in relation to fruit body formation in the mycelium of Lentinula edodes on a sawdust-based substrate. Mycoscience, 41, 149–153. doi:10.1007/BF02464324.
Chen, S., Ge, W., & Buswell, J. A. (2004). Biochemical and molecular characterization of a laccase from the edible straw mushroom, Volvariella volvacea. European Journal of Biochemistry, 271, 318–328. doi:10.1046/j.1432-1033.2003.03930.x.
Wood, D. (1980). Production, purification and properties of extracellular laccase of Agaricus bisporus. Journal of General Microbiology, 117, 327–338.
De Vries, O. M. H., Kooistra, W. H. C. F., & Wessels, G. M. (1986). Formation of extracellular laccase by Schizophyllum commune dikaryon. Holzforschung, 46, 135–147.
Aisemberg, G. O., Grorewold, E., Taccioli, G. E., & Judewicz, N. (1989). A major transcript in the response of Neurospora crassa to protein synthesis inhibition by cycloheximide. Experimental Mycology, 13, 121–128. doi:10.1016/0147-5975(89)90017-0.
Rigling, D., & Van Alfen, N. K. (1991). Regulation of laccase biosynthesis in plant pathogenic fungus Cryphonectria parasitica by double stranded RNA. Journal of Bacteriology, 173, 8000–8003.
Kim, D. H., Rigling, D., Zhang, L., & Van Alfen, N. K. (1995). A new extracellular laccase of Cryphonectria parasitica is revealed by deletion of Lac 1. Molecular Plant–Microbe Interactions, 8, 259–266.
Binz, T., & Canevascini, G. (1996). Differential production of extracellular laccase by Dutch elm disease pathogen Ophiostoma ulmi and O. novo-ulmi. Mycological Research, 100, 1060–1064. doi:10.1016/S0953-7562(96)80213-9.
Brasier, C. M. (1991). Ophiostoma novo-ulmi species causative agent of current Dutch elm disease pandemic. Mycopathologia, 115, 151–161. doi:10.1007/BF00462219.
Waterman, S. R., Hacham, M., Panepinto, J., Hu, G., Shin, S., & Williamson, P. R. (2007). Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infection and Immunity, 75, 714–722. doi:10.1128/IAI.01351-06.
Litvintseva, A. P., & Henson, J. M. (2002). Characterization of two laccase genes of Gaeumannomyces graminis var. graminis and their differential transcription in melanin mutants and wild type. Mycological Research, 106, 808–814. doi:10.1017/S0953756202005841.
Ander, P., & Eriksson, K. E. (1976). The importance of phenol oxidase activity in lignin degradation by the white rot fungus Sporotrichum pulverulentum. Archives of Microbiology, 109, 1–8. doi:10.1007/BF00425105.
Bermek, H., Li, K., & Eriksson, K. E. (1998). Laccase-less mutants of the white rot fungus Pycnoporus cinnabarinus cannot delignify kraft pulp. Journal of Biotechnology, 66, 117–124. doi:10.1016/S0168-1656(98)00091-1.
Garzillo, A. M. V., Colao, M. C., Caruso, C., Caporale, C., Celletti, D., & Buonocore, V. (1998). Laccase from the white rot fungus Trametes trogii. Applied Microbiology and Biotechnology, 49, 545–551. doi:10.1007/s002530051211.
Bourbonnais, R., & Paice, M. G. (1990). Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Letters, 267, 99–102. doi:10.1016/0014-5793(90)80298-W.
Srinivasan, C., D’souza, T. M., Boominathan, K., & Reddy, C. A. (1995). Demonstration of laccase in the white rot basidiomycete Phanerochaete chrysosporium BKM-F-1767. Applied and Environmental Microbiology, 61, 4274–4277.
Sethuraman, A., Akin, D. E., & Eriksson, K. E. L. (1999). Production of ligninolytic enzymes and synthetic lignin mineralization by birds nest fungus Cyathus stercoreus. Applied Microbiology and Biotechnology, 52, 689–697. doi:10.1007/s002530051580.
Arora, D. S., & Sandhu, D. K. (1985). Laccase production and wood degradation by a white rot fungus Daedalea flavida. Enzyme and Microbial Technology, 7, 405–408. doi:10.1016/0141-0229(85)90131-0.
Arora, D. S., & Gill, P. K. (2001). Effects of various media and supplements on laccase production by some white rot fungi. Bioresource Technology, 77, 89–91. doi:10.1016/S0960-8524(00)00114-0.
Arora, D. S., & Sandhu, D. K. (1987). Decomposition of angiospermic wood sawdust and laccase production by two Pleurotus species. Journal of Basic Microbiology, 27, 179–184. doi:10.1002/jobm.3620270402.
Fortina, M. G., Acquati, A., Rossi, P., Manachini, P. L., & Di Gennaro, C. (1996). Production of laccase by Botrytis cinerea and fermentation studies with strain 226. Journal of Industrial Microbiology, 17, 69–72. doi:10.1007/BF01570044.
Dhaliwal, R. P. S., Garcha, H. S., & Khanna, P. K. (1992). High laccase producing mutants of Pleurotus florida. World Journal of Microbiology & Biotechnology, 8, 39–41. doi:10.1007/BF01200681.
Nagai, M., Sato, T., Watanabe, H., Saito, K., Kawata, M., & Enei, H. (2002). Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Applied Microbiology and Biotechnology, 60, 327–335. doi:10.1007/s00253-002-1109-2.
Reader, U., & Broda, P. (1985). Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology, 1, 17–20. doi:10.1111/j.1472-765X.1985.tb01479.x.
Kim, Y., Yeo, S., Song, H. G., & Choi, H. T. (2008). Enhanced expression of laccase during the degradation of endocrine disrupting chemicals in Trametes versicolor. Journal of Microbiology (Seoul, Korea), 46, 402–407. doi:10.1007/s12275-007-0236-y.
Liu, W., Chao, Y., Liu, S., Bao, H., & Qian, S. (2003). Molecular cloning and characterization of a laccase gene from the basidiomycete Fomes lignosus and expression in Pichia pastoris. Applied Microbiology and Biotechnology, 63, 174–181. doi:10.1007/s00253-003-1398-0.
Susla, M., Novotnỳ, C., & Svobodová, K. (2007). The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures. Bioresource Technology, 98, 2109–2115. doi:10.1016/j.biortech.2006.08.007.
Ong, E., Pollock, W. B. R., & Smith, M. (1997). Cloning and sequence analysis of two laccase complementary DNAs from the ligninolytic basidiomycete Trametes versicolor. Gene, 196, 113. doi:10.1016/S0378-1119(97)00215-1.
Yaver, D. S., & Golightly, E. J. (1996). Cloning and characterization of three laccase genes from the white rot basidiomycetes Trametes villosa: Genomic organization of the laccase gene family. Gene, 181, 95–102. doi:10.1016/S0378-1119(96)00480-5.
Wahleithner, J. A., Xu, F., Brown, K. M., Brown, S. H., Golightly, E. J., Halkier, T., et al. (1996). The identification and characterization of four laccase from the plant pathogenic fungus Rhizoctonia solani. Current Genetics, 29, 395–403. doi:10.1007/BF02208621.
Moldes, D., Lorenzo, M., & Sanroman, M. A. (2004). Different proportions of laccase isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic waste. Biotechnology Letters, 26, 327–330. doi:10.1023/B:BILE.0000015452.40213.bf.
Mansur, M., Suarez, T., Fernandez-Larrea, J., Brizuela, M. A., & Gonzalez, A. E. (1997). Identification of a laccase gene family in the new lignin degrading basidiomycete CECT 20197. Applied and Environmental Microbiology, 63, 2637–2646.
Okano, K., Iida, Y., Samurai, M., Prasetya, B., Usagawa, T., & Watanabe, T. (2006). Comparison of in-vitro digestibility and chemical composition among sugarcane bagasses treated by four white rot fungi. Animal Science Journal, 77, 308–313. doi:10.1111/j.1740-0929.2006.00353.x.
Arora, D. S., & Sharma, R. K. (2009). Comparative ligninolytic potential of Phlebia species and their role in improvement of in vitro digestibility of wheat straw. Journal of Animal and Feed Sciences, 18, 151–161.
Kirk, T. K. (1983). Degradation and conversion of lignocelluloses. In J. E. Smith, D. R. Berry & B. Kristiansen (Eds.), The filamentous fungi (Vol. 4, pp. 266–295). London: Edward Arnold.
Kristensen, J. B., Thygesen, L. G., Felby, C., Jørgensen, H., & Elder, T. (2008). Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnology for Biofuels, 1, 5. doi:10.1186/1754-6834-1-5.
Jonsson, L. J., Palmqvist, E., Nilvebrant, N. O., & Hahn-Hägerdal, B. (1998). Detoxification of wood hydrolysates with laccase and peroxidase from the white rot fungus Trametes versicolor. Applied Microbiology and Biotechnology, 49, 691–697. doi:10.1007/s002530051233.
Widsten, P., & Kandelbauer, A. (2008). Laccase applications in the forest products industry: A review. Enzyme and Microbial Technology, 42, 293–307. doi:10.1016/j.enzmictec.2007.12.003.
Mendonça, R. T., Jara, J. F., González, V., Elissetche, J. P., & Freer, J. (2008). Evaluation of the white-rot fungi Ganoderma australe and Ceriporiopsis subvermispora in biotechnological applications. Journal of Industrial Microbiology & Biotechnology, 35, 1323–1330. doi:10.1007/s10295-008-0414-x.
Wong, K. K. Y., Richardson, J. D., & Mansfield, S. D. (2000). Enzymatic treatment of mechanical pulp fibres for improving paper making properties. Biotechnology Progress, 16, 1025–1029. doi:10.1021/bp000064d.
Bajpai, P. (1999). Application of enzymes in the pulp and paper industry. Biotechnology Progress, 15, 147–157. doi:10.1021/bp990013k.
Scott, G. M., Lentz, M., Akhtar, M., Sykes, M., & Abubakr, S. (1995). Environmental aspects of biosulfite pulping. Proceedings of 1995 international environmental conference (pp. 1155–1161). Atlanta: TAPPI.
Call, H. P. (1994). Process for modifying, breaking down or bleaching lignin, materials containing or like substances. World patent application WO 94/29510.
Wesenberg, D., Kyriakides, I., & Agathos, S. N. (2003). White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22, 161–187. doi:10.1016/j.biotechadv.2003.08.011.
Liu, W., Chao, Y., Yang, X., Bao, H., & Qian, S. (2004). Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. Journal of Industrial Microbiology & Biotechnology, 31, 127–132. doi:10.1007/s10295-004-0123-z.
Swamy, J., & Ramsay, J. A. (1999). Effects of Mn2+ and NH +4 concentration on laccase and MnP production and amaranth decoloration by Trametes versicolor. Applied Microbiology and Biotechnology, 51, 391–396. doi:10.1007/s002530051408.
Kirby, N., Marchant, R. M., & Mullan, G. (2000). Decolorization of synthetic textile dyes by Phlebia tremellosa. FEMS Microbiology Letters, 188, 93–96. doi:10.1111/j.1574-6968.2000.tb09174.x.
Gill, P. K., Arora, D. S., & Chander, M. (2002). Biodecolorisation of azo and triphenyl-methane dyes by Dichomitus squalens and Phlebia spp. Journal of Industrial Microbiology & Biotechnology, 28, 201–203. doi:10.1038/sj.jim.7000222.
Chander, M., & Arora, D. S. (2007). Evaluation of some white rot fungi for their potential to decolorise industrial dyes. Dyes and Pigments, 72, 192–198. doi:10.1016/j.dyepig.2005.08.023.
Murugesan, K., Yang, I. H., Kim, Y. M., Jeon, J. R., & Chang, Y. S. (2009). Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Applied Microbiology and Biotechnology, 82, 341–350. doi:10.1007/s00253-008-1819-1.
Ciullini, I., Tilli, S., Scozzafava, A., & Brigant, F. (2008). Fungal laccase, cellobiose dehydrogenase, and chemical mediators: Combined actions for the decolorization of different classes of textile dyes. Bioresource Technology, 99, 7003–7010. doi:10.1016/j.biortech.2008.01.019.
Cantarelli, C., Brenna, O., Giovanelli, G., & Rossi, M. (1989). Beverage stabilization through enzymatic removal of phenolics. Food Biotechnology, 3, 203–214.
Minussi, R. C., Pastore, G. M., & Duran, N. (2002). Potential applications of laccase in the food industry. Trends in Food Science & Technology, 13, 205–216. doi:10.1016/S0924-2244(02)00155-3.
Giovanelli, G., & Ravasini, G. (1993). Apple juice stabilization by combined enzyme membrane filtration process. Lebensmittel-Wissenschaft + Technologie, 26, 1–7. doi:10.1006/fstl.1993.1001.
Rubilar, O., Diez, M. C., & Gianfreda, L. (2008). Transformation of chlorinated phenolic compounds by white rot fungi. Critical Reviews in Environmental Science and Technology, 38, 227–268. doi:10.1080/10643380701413351.
Raghukumar, C., D'Souza-Ticlo, D., & Verma, A. K. (2008). Treatment of colored effluents with lignin-degrading enzymes: An emerging role of marine-derived fungi. Critical Reviews in Microbiology, 34, 189–206. doi:10.1080/10408410802526044.
D'souza, T. D., Tiwari, R., Sah, A. K., & Raghukumar, C. (2006). Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzyme and Microbial Technology, 38, 504–511. doi:10.1016/j.enzmictec.2005.07.005.
Gianfreda, L., Iamarino, G., Scelza, R., & Rao, M. A. (2006). Oxidative catalysts for the transformation of phenolic pollutants: A brief review. Biocatalysis and Biotransformation, 24, 177–187. doi:10.1080/10242420500491938.
Minussi, R. C., Pastore, M. G., & Duran, N. (2007). Laccase induction in fungi and laccase/N-OH mediator systems applied in paper mill effluent. Bioresource Technology, 98, 158–164. doi:10.1016/j.biortech.2005.11.008.
Minussi, R. C., Miranda, M. A., Silva, J. A., Ferreira, C. V., Aoyama, H., Marangoni, S., et al. (2007). Purification, characterization and application of laccase from Trametes versicolor for colour and phenolic removal of olive mill wastewater in the presence of 1-hydroxybenzotriazole. African Journal of Biotechnology, 6, 1248–1254.
Sampedro, I., Cajthaml, T., Marinari, S., Petruccioli, M., Grego, S., & D'Annibale, A. (2009). Organic matter transformation and detoxification in dry olive mill residue by the saprophytic fungus Paecilomyces farinosus. Process Biochemistry, 44, 216–225. doi:10.1016/j.procbio.2008.10.016.
Villaasenor, F., Lorea, O., Campero, A., & Viniegra-Gonzalez, G. (2004). Oxidation of dibenzothiophene by laccase or hydrogen peroxide and deep desulfurization of diesel fuel by the latter. Fuel Processing Technology, 86, 49–59. doi:10.1016/j.fuproc.2003.12.007.
Xu, P., Yu, B., Li, F. L., Cai, X. F., & Ma, C. Q. (2006). Microbial degradation of sulfur, nitrogen and oxygen heterocycles. Trends in Microbiology, 14, 398–405. doi:10.1016/j.tim.2006.07.002.
Catcheside, D. E. A., & Ralph, J. P. (1999). Biological processing of coal. Applied Microbiology and Biotechnology, 52, 16–24. doi:10.1007/s002530051482.
Keum, Y. S., & Li, Q. X. (2004). Copper dissociation as a mechanism of fungal laccase denaturation by humic acid. Applied Microbiology and Biotechnology, 64, 588–592. doi:10.1007/s00253-003-1460-y.
Fakoussa, R. M., & Frost, P. J. (1999). In-vivo decolorization of coal derived humic acids by laccase excreting fungus Trametes versicolor. Applied Microbiology and Biotechnology, 52, 60–65. doi:10.1007/s002530051487.
Ryan, P. J., Gross, D., Owen, W. J., & Loani, T. L. (1981). The metabolism of chlorotoluron, diuron and CGA 43057 in tolerant and susceptible plants. Pesticide Biochemistry and Physiology, 16, 213–221. doi:10.1016/0048-3575(81)90055-9.
Jolivalt, C., Raynal, A., Caminade, E., Kokel, B., Le Goffic, F., & Mougin, C. (1999). Transformation of N,N′-dimethyl-N-(hydroxyphenol) urea by laccase from white rot fungus Trametes versicolor. Applied Microbiology and Biotechnology, 51, 676–681. doi:10.1007/s002530051451.
Kamei, I., & Kondo, R. (2006). Simultaneous degradation of commercially produced CNP herbicide and of contaminated dioxin by treatment using the white-rot fungus Phlebia brevispora. Chemosphere, 65, 1221–1227. doi:10.1016/j.chemosphere.2006.03.030.
Pozdnyakova, N. N., Rodakiewicz-Nowak, J., Turkovskaya, O. V., & Haber, J. (2006). Oxidative degradation of polyaromatic hydrocarbons catalyzed by blue laccase from Pleurotus ostreatus D1 in the presence of synthetic mediators. Enzyme and Microbial Technology, 39, 1242–1249. doi:10.1016/j.enzmictec.2006.03.009.
Duran, N., & Esposito, E. (2000). Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Applied Catalysis B: Environmental, 28, 83–99. doi:10.1016/S0926-3373(00)00168-5.
Pointing, S. B. (2001). Feasibility of bioremediation by white rot fungi. Applied Microbiology and Biotechnology, 57, 20–33. doi:10.1007/s002530100745.
Couto, S. R., & Toca-Herrera, J. L. (2006). Laccases in the textile industry. Biotechnology and Molecular Biology Review, 4, 117–122.
Selinheimo, E., Kruus, K., Buchert, J., Hopia, A., & Autio, K. (2006). Effects of laccase, xylanase and their combination on the rheological properties of wheat dough. Journal of Cereal Science, 43, 152–159. doi:10.1016/j.jcs.2005.08.007.
Selinheimo, E., Autio, K., Kruus, K., & Buchert, J. (2007). Elucidating the mechanism of laccase and tyrosinase in wheat bread making. Journal of Agricultural and Food Chemistry, 55, 6357–6365. doi:10.1021/jf0703349.
Bhalerao, U. T., Muralikrishna, C., & Rani, B. R. (1994). Laccase enzyme catalyzed efficient synthesis of 3-substituted 1,2,4-triazolo(4, 3-b)(4, 1, 2)benzothiazidine-8-ones. Tetrahedron, 50, 4019–4024. doi:10.1016/S0040-4020(01)89677-0.
Mikolasch, A., Hammer, E., Jonas, U., Popowski, K., Stielow, A., & Schauer, F. (2002). Synthesis of 3-(3,4-dihydroxy-phenyl)-propionic acid derivatives by N-coupling of amines using laccase. Tetrahedron, 58, 7589–7593. doi:10.1016/S0040-4020(02)00872-4.
Lang, G., & Cotteret, J. (1999). Hair dye composition containing a laccase. (L’Oreal, Fr.). International Patent Application, WO9936036.
Golz-Berner, K., Walzel, B., Zastrow, L., & Doucet, O. (2004). Cosmetic and dermatological preparation containing copper-binding proteins for skin lightening. International Patent Application, WO2004017931.
Wang, H. X., & Ng, T. B. (2004). Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochemical and Biophysical Research Communications, 315, 450–454. doi:10.1016/j.bbrc.2004.01.064.
Haghighi, B., Gorton, L., Ruzgas, T., & Jönsson, L. J. (2003). Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Analytica Chimica Acta, 487, 3–14. doi:10.1016/S0003-2670(03)00077-1.
Yaropolov, A. I., Skorobogatko, O. V., Vartanov, S. S., & Varfolomeyev, S. D. (1994). Laccase: Properties, catalytic mechanism and applicability. Applied Biochemistry and Biotechnology, 49, 257–280. doi:10.1007/BF02783061.
Cordi, L., Minussi, R. C., Freire, R. S., & Durán, N. (2007). Fungal laccase: Copper induction, semi-purification, immobilization, phenolic effluent treatment and electrochemical measurement. African Journal of Biotechnology, 6, 1255–1259.
Schmid, R. D., & Urlacher, V. B. (2007). Modern biooxidation: Enzymes, reactions and applications (p. 53). Weinheim: Wiley.
Duran, N., Rosa, M. A., D'Annibale, A., & Gianfreda, L. (2002). Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzyme and Microbial Technology, 31, 907–931. doi:10.1016/S0141-0229(02)00214-4.
Messerschmidt, A. (1994). Blue copper oxidases. Advances in Inorganic Chemistry, 40, 121–185. doi:10.1016/S0898-8838(08)60183-X.
Bonnen, A. M., Anton, L. H., & Orth, A. B. (1994). Lignin-degrading enzymes of the commercial button mushroom, Agaricus bisporus. Applied and Environmental Microbiology, 60, 960–965.
De Jong, E., Field, J. A., & De Bont, J. A. M. (1992). Evidence for a new extracellular peroxidase. Manganese inhibited peroxidase from the white rot fungus Bjerkandera sp. BOS55. FEBS Letters, 99, 107–110. doi:10.1016/0014-5793(92)80111-S.
Ruttimann-Johnson, C., Salas, L., Vicuna, R., & Kirk, T. K. (1993). Extracellular enzyme production and synthetic lignin mineralization by Ceriporiopsis subvermispora. Applied and Environmental Microbiology, 59, 1792–1797.
Morita, Y., Yamashita, H., Mikami, B., Iwamoto, H., Aibara, S., Terada, M., et al. (1988). Purification, crystallization and characterization of peroxidase from Coprinus cinereus. Journal of Biochemistry, 103, 693–699.
Heinzkill, M., Bech, L., Halkier, T., Schneider, P., & Anke, T. (1998). Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Applied and Environmental Microbiology, 64, 1601–1606.
Vyas, B. R. M., Volc, J., & Sasek, V. (1994). Ligninolytic enzymes of selected white rot fungi cultivated on wheat straw. Folia Microbiologica, 39, 235–240. doi:10.1007/BF02814655.
Vasdev, K., & Kuhad, R. C. (1994). Induction of laccase production in Cyathus bulleri under shaking and static culture conditions. Folia Microbiologica, 39, 326–330. doi:10.1007/BF02814322.
Arora, D. S., & Gill, P. K. (2000). Laccase production by some white rot fungi under different nutritional conditions. Bioresource Technology, 73, 283–285. doi:10.1016/S0960-8524(99)00141-8.
Haars, A., & Huttermann, A. (1983). Laccase induction in the white rot fungus Heterobasidion annosum (Fr.) Bref. (Fomes annosus Fr. Cooke). Archives of Microbiology, 134, 309–313. doi:10.1007/BF00407808.
Nerud, F., Zouchova, Z., & Misurcova, Z. (1991). Ligninolytic properties of different white rot fungi. Biotechnology Letters, 13, 657–660. doi:10.1007/BF01086322.
Farnet, A. M., Criquet, S., Tagger, S., Gil, G., & Le Petit, J. (2000). Purification, partial characterization and reactivity with aromatic compounds of two laccases from Marasmius quercophilus strain 17. Canadian Journal of Microbiology, 46, 1–6. doi:10.1139/cjm-46-3-189.
Vares, T., Niemenmaa, O., & Hatakka, A. (1994). Secretion of ligninolytic enzymes and mineralization of 14C ring labeled synthetic lignin by three Phlebia tremellosa strains. Applied and Environmental Microbiology, 60, 569–575.
Martinez, A. T., Camarero, S., Guillen, F., Gutiérrez, A., Muñoz, C., Varela, E., et al. (1994). Progress in biopulping of non-woody materials: Chemicals, enzymatic and ultrastructural aspects of wheat straw delignification with ligninolytic fungi from genus Pleurotus. FEMS Microbiology Reviews, 13, 265–273.
Bourbonnais, R., & Paice, M. G. (1988). Veratryl alcohol oxidases from the lignin-degrading basidiomycete Pleurotus sajor-caju. The Biochemical Journal, 255, 445–450.
Huttermann, A., Milstein, O., Nicklas, B., Trojanowski, J., Haars, A., & Kharazipour, A. (1989). Enzymatic modification of lignin for technical use. In W. G. Glasser & S. Sarkanen (Eds.), Lignin—properties and materials. ACS symposium series (Vol. 397 , pp. 361–370). Washington, DC: American Chemical Society.
Galliano, H., Gas, G., & Seris, J. L. (1991). Lignin degradation by Rigidoporus lignosus involves synergistic action of two oxidizing enzymes: MnP and laccase. Enzyme and Microbial Technology, 13, 478–482. doi:10.1016/0141-0229(91)90005-U.
Dong, J. L., & Zhang, Y. Z. (2004). Purification and characterization of two laccase isoenzymes from a ligninolytic fungus Trametes gallica. Preparative Biochemistry & Biotechnology, 34, 179–194. doi:10.1081/PB-120030876.
Fasola, T. R., Gbolagade, J. S., & Fasidi, I. O. (2007). Nutritional requirements of Volvariella. speciosa (Fr.Ex.Fr.) Singer, a Nigerian edible mushroom. Food Chemistry, 100, 904–908. doi:10.1016/j.foodchem.2005.10.061.
Mayer, A. M., & Harel, E. (1979). Polyphenol oxidases in plants. Phytochemistry, 18, 193–215. doi:10.1016/0031-9422(79)80057-6.
Kiiskinen, L. L., Viikari, L., & Kruus, K. (2002). Purification and characterization of a novel laccase from the ascomycete Melanocarpus albomyces. Applied Microbiology and Biotechnology, 59, 198–204. doi:10.1007/s00253-002-1012-x.
Durrens, P. (1981). The phenoloxidase of the ascomycete Podospora anserina: The three forms of major laccase activity. Archives of Microbiology, 130, 121–124. doi:10.1007/BF00411062.
Kurtz, M. B., & Champe, S. P. (1982). Purification and characterization of the conidial laccase of Aspergillus nidulans. Journal of Bacteriology, 151, 1338–1345.
Slomczynski, D., Nakas, J. P., & Tanenbaum, S. W. (1995). Production and characterization of laccase from Botrytis cinerea 61-34. Applied and Environmental Microbiology, 61, 907–912.
Thakker, G., Evans, C. S., & Rao, K. K. (1992). Purification and characterization of laccase from Monocillium indicum. Applied Microbiology and Biotechnology, 37, 321–323. doi:10.1007/BF00210986.
Lee, K. H., Wi, S. G., Singh, A. P., & Kim, Y. S. (2004). Micromorphological characteristics of decayed wood and laccase produced by brown rot fungus Coniophora puteana. Journal of Wood Science, 50, 291–284. doi:10.1007/s10086-003-0558-2.
Kirk, T. K., & Shimada, M. (1985). Lignin biodegradation: The microorganisms involved and the physiology and biochemistry of degradation by white-rot fungi. In T. Higuchied (Ed.), Biosynthesis and bio degradation of wood components (pp. 579–605). Orlando: Academic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh Arora, D., Kumar Sharma, R. Ligninolytic Fungal Laccases and Their Biotechnological Applications. Appl Biochem Biotechnol 160, 1760–1788 (2010). https://doi.org/10.1007/s12010-009-8676-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8676-y