Abstract
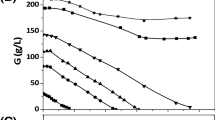
Glycerol is a renewable resource for it is formed as a byproduct during biodiesel production. Because of its large volume production, it seems to be a good idea to develop a technology that converts this waste into products of high value, for example, to 1,3-propanediol (1,3-PD). We suggested an enzymatic bioconversion in a membrane reactor in which the NAD coenzyme can be regenerated, and three key enzymes are retained by a 10-kDa ultrafilter membrane. Unfortunately, some byproducts also formed during successful glycerol to 1,3-PD bioconversion runs, as we used crude enzyme solution of Klebsiella pneumoniae. To study the possibilities to avoid this byproduct formation, we built a mathematical description of this system. The model was also used for simulation bioconversions of high glycerol concentration with and without elimination of byproduct formation and of continuous operation.









Similar content being viewed by others
Abbreviations
- 1,3-PD:
-
1,3-propanediol
- DHA:
-
1,3-dihydroxyacetone
- GDH:
-
glycerol dehydrogenase enzyme
- GDHt:
-
glycerol dehydratase enzyme
- PDOR:
-
1,3-propanediol oxydoreductase
- DHAK:
-
dihydroxyacetone kinase
- TPI:
-
triose-phosphate isomerase
- GAPD:
-
glyceraldehyde-3-phosphate dehydrogenase
- PGK:
-
phosphoglycerate kinase
- PGM:
-
phosphoglycerate mutase
- PPH:
-
phosphopyruvate hydratase
- PK:
-
pyruvate kinase
- PS:
-
pyruvate synthase
- PAT:
-
phosphate acetyltransferase
- PTA:
-
phospholipid-translocating ATPase
- LDH:
-
lactate-dehydrogenase
- LMO:
-
lactate 2-monooxygenase
- BB:
-
“black-box”
- Clha:
-
chloro-3-hydroxyacetone
References
Dunn-Coleman, N. S., Gatenby, A. A., Valle, F. (1998). Methods for the production of 1,3-propanediol by recombinant organisms. World Patent WO9821339.
Németh, Á., Kupcsulik, B., & Sevella, B. (2003). 1,3-Propanediol oxidoreductase production with Klebsiella pneumoniae DSM2026. World Journal of Microbiology and Biotechnology, 19(7), 659–663.
Johnson, E. A., & Lin, C. C. (1987). Klebsiella pneumoniae 1,3-Propanediol:NAD+ Oxidoreductase. Journal of Bacteriology, 169(5), 2050–2054.
Toraya, T., Ushio, K., Fukui, S., & Hogenkamp, P. C. (1977). Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of coenzyme. Journal of Biological Chemistry, 252(3), 963–970.
Biebl, H., Menzel, K., Zeng, A. P., & Deckwer, W. D. (1999). Microbial production of 1,3-propanediol. Applied Microbiology and Biotechnology, 52(3), 289–297.
Mori, K., Tobimatsu, T., Hara, T., & Toraya, T. (1997). Characterization, sequencing, and expression of the genes encoding a reactivating factor for glycerol-inactivated adenosylcobalamin-dependent diol dehydratase. Journal of Biological Chemistry 272(51), 32034–32041.
McGregor, W. J., Phillips, J., Suelter, C. H. (1974). Purification and kinetic characterization of a monovalent cation-activated glycerol dehydrogenase from Aerobacter aerogenes. Journal of Biological Chemistry, 249(10), 3132–3139.
Garcia-Alles, L. F., Siebold, C., Nyffeler, T. L., Flukiger-Bruhwiler, K., Schneider, P., Burgi, H. B., et al. (2004). Phosphoenolpyruvate and ATP-dependent dihydroxyacetone kinases: Covalent substrate-binding and kinetic mechanism. Biochemistry, 43, 13037–13045.
Yamanishi, M., Yunoki, M., Tobimatsu, T., Sato, H., Matsui, J., Dokiya, A., et al. (2002). The crystal structure of coenzyme B12-dependent glycerol dehydratase in complex with cobalamin and propane-1,2-diol. European Journal of Biochemistry, 269(18), 4484–4494.
Johnson, E. A., Burke, S. K., Forage, R. G., & Lin, E. C. (1984). Purification and properties of dihydroxyacetone kinase from Klebsiella pneumoniae. Journal of Bacteriology, 160(1), 55–60.
Cornish Bowden, A. (2004). Fundamentals of Enzyme Kinetics, 3rd edn. London, UK: Portland.
Berglund, O., & Eckstein, F. (1972). ATP and dATP-substituted agaroses and the purification of ribonucleotide reductase. Journal of Biological Chemistry, 224(7276), 253–261.
Bückmann, A. F. (1981). An efficient synthesis of High-Molecular-Weight NAD(H) derivatives suitable for continuous operation with coenzyme-dependent enzyme systems. Journal of Applied Biochemistry, 3, 301–315.
Obon, J. M., Manjon, A., & Iborra, J. L. (1998). Retention and regeneration of native NAD(H) in noncharged ultrafiltration membrane reactors: application to l-lactate and gluconate production. Biotechnology and Bioengineering, 57(5), 510–517.
Reynaud, C., Sarçabal, P., Meynial-Salles, I., Croux, C., & Soucaille, P. (2003). Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Applied Biological Sciences, 100(9), 5010–5015.
Acknowledgment
We are thankful to OTKA T032015 and NKFP-3/A/0035/2002 for the financial support of our work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Németh, Á., Sevella, B. Development of a New Bioprocess for Production of 1,3-propanediol I.: Modeling of Glycerol Bioconversion to 1,3-propanediol with Klebsiella pneumoniae Enzymes. Appl Biochem Biotechnol 144, 47–58 (2008). https://doi.org/10.1007/s12010-007-0040-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-0040-5