Abstract
Formulations of bio-based poly(cyclic carbonates) and amines using cooperative catalysis were studied to produce non-isocyanate polyurethanes (NIPUs). Concerns on the use of isocyanates as starting materials for polyurethanes (PUs) have risen due to their effects on human health after exposure and also because their synthesis involves the use of phosgene. Polyurethanes are highly versatile materials used in widespread industries such as automotive, building, construction, and packaging. They have also been used as flexible and rigid foams, adhesives, coatings, thermoplastic, or thermoset materials. Traditionally, PUs are synthesized from polyols and polyisocyanates. In order to circumvent the concerns, much research has been devoted to exploring alternative approaches to the synthesis of PUs. NIPU synthesis using cyclic carbonates and amines has gained popularity as one of the new approaches. In this study, novel bio-based resins were synthesized by converting epoxidized sucrose soyate into carbonated sucrose soyate (CSS) under supercritical conditions. Initial studies have shown promise in systems where CSS is crosslinked with multifunctional amines generating coatings with good solvent resistance. This work focused on studying the effect of catalysts and developing formulations of bio-based non-isocyanate polyurethane coatings.





















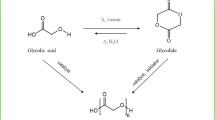
Reproduced from literature Ref. 50
Similar content being viewed by others
References
Demharter, A, “Polyurethane Rigid Foam, A Proven Thermal Insulating Material for Applications Between + 130 C and − 196 C.” Cryogenics, 38 (1) 113–117 (1998)
Krol, P, “Synthesis Methods, Chemical Structures and Phase Structures of Linear Polyurethanes. Properties and Applications of Linear Polyurethanes in Polyurethane Elastomers, Copolymers and Ionomers.” Prog. Mater. Sci., 52 (6) 915–1015 (2007)
Santerre, JP, Woodhouse, K, Laroche, G, Labow, RS, “Understanding the Biodegradation of Polyurethanes: From Classical Implants to Tissue Engineering Materials.” Biomaterials, 26 (35) 7457–7470 (2005)
Zdrahala, RJ, Zdrahala, IJ, “Biomedical Applications of Polyurethanes: A Review of Past Promises, Present Realities, and a Vibrant Future.” J. Biomater. Appl., 14 (1) 67–90 (1999)
Verschoor, L, Verschoor, AH, “Nonoccupational and Occupational Exposure to Isocyanates.” Curr. Opin. Pulm. Med., 20 (2) 199–204 (2014)
Lockey, JE, Redlich, CA, Streicher, R, Pfahles-Hutchens, A, Hakkinen, PJ, Ellison, GL, Harber, P, Utell, M, Holland, J, Comai, A, “Isocyanates and Human Health: Multi-stakeholder Information Needs and Research Priorities.” J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med., 57 (1) 44 (2015)
Rokicki, G, Parzuchowski, PG, Mazurek, M, “Non-isocyanate Polyurethanes: Synthesis, Properties, and Applications.” Polym. Adv. Technol., 26 (7) 707–761 (2015)
Figovsky, OL, Leykin, AD, Shapovalov, LL, “Non-isocyanate Polyurethanes-Yesterday, Today and Tomorrow.” Int. Sci. J. Altern. Energy Ecol., 3–4 95–108 (2016)
Maisonneuve, L, Lamarzelle, OA, Rix, E, Grau, E, Cramail, H, “Isocyanate-Free Routes to Polyurethanes and Poly (Hydroxy Urethane)s.” Chem. Rev., 115 (22) 12407–12439 (2015)
Kathalewar, MS, Joshi, PB, Sabnis, AS, Malshe, VC, “Non-isocyanate Polyurethanes: From Chemistry to Applications.” RSC Adv., 3 (13) 4110–4129 (2013)
Delebecq, E, Pascault, J-P, Boutevin, B, Ganachaud, FO, “On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane.” Chem. Rev., 113 (1) 80–118 (2012)
Guan, J, Song, Y, Lin, Y, Yin, X, Zuo, M, Zhao, Y, Tao, X, Zheng, Q, “Progress in Study of Non-isocyanate Polyurethane.” Ind. Eng. Chem. Res., 50 (11) 6517–6527 (2011)
Farhadian, A, Afshani, G, Babapour, M, Babaei Miyardan, A, Nabid, MR, Safari, N, “A Facile and Green Route for Conversion of Bifunctional Epoxide and Vegetable Oils to Cyclic Carbonate: A Green Route to CO2 Fixation.” ChemistrySelect, 2 (4) 1431–1435 (2017)
Büttner, H, Steinbauer, J, Wulf, C, Dindaroglu, M, Schmalz, HG, Werner, T, “Organocatalyzed Synthesis of Oleochemical Carbonates from CO2 and Renewables.” ChemSusChem, 10 (6) 1076–1079 (2017)
Guzmán, AF, Echeverri, DA, Rios, LA, “Carbonation of Epoxidized Castor Oil: A New Bio-based Building Block for the Chemical Industry.” J. Chem. Technol. Biotechnol., 92 (5) 1104–1110 (2017)
Poussard, L, Mariage, J, Grignard, B, Detrembleur, C, Jérôme, C, Calberg, C, Heinrichs, B, De Winter, J, Gerbaux, P, Raquez, JM, “Non-isocyanate Polyurethanes from Carbonated Soybean Oil Using Monomeric or Oligomeric Diamines to Achieve Thermosets or Thermoplastics.” Macromolecules, 49 (6) 2162–2171 (2016)
Javni, I, Hong, DP, Petrović, ZS, “Soy-Based Polyurethanes by Nonisocyanate Route.” J. Appl. Polym. Sci., 108 (6) 3867–3875 (2008)
Tamami, B, Sohn, S, Wilkes, GL, “Incorporation of Carbon Dioxide into Soybean Oil and Subsequent Preparation and Studies of Nonisocyanate Polyurethane Networks.” J. Appl. Polym. Sci., 92 (2) 883–891 (2004)
Howie JK, Schaefer JJ, Trout JE, “Synthesis of Polyol Medium Fatty Acid Polyesters.” US Patent 6,995,232, 2006
Corrigan PJ, “Synthesis of Polyol Fatty Acid Polyesters.” US Patent 6,620,952, 2003
Schaefer JJ, Trout JE, “Synthesis of Purified, Partially Esterified Polyol Polyester Fatty Acid Compositions.” US Patent 6,887,947, 2005
Webster, DC, Sengupta, PP, Chen, Z, Pan, X, Paramarta, A., "Highly Functional Epoxidized Resins and Coatings." US Patent 9,096,773, 2015
Pan, X, Webster, DC, “New Biobased High Functionality Polyols and Their Use in Polyurethane Coatings.” ChemSusChem, 5 (2) 419–429 (2012)
Paramarta, A, Pan, X, Webster, DC, “Highly Functional Acrylated Biobased Resin System.” Radtech Rep., 1 26–32 (2013)
Nelson, TJ, Bultema, L, Eidenschink, N, Webster, DC, “Bio-based High Functionality Polyols and Their Use in 1 K Polyurethane Coatings.” J. Renew. Mater., 1 (2) 141–153 (2013)
Kovash, CS, Pavlacky, E, Selvakumar, S, Sibi, MP, Webster, DC, “Thermoset Coatings from Epoxidized Sucrose Soyate and Blocked, Bio-Based Dicarboxylic Acids.” ChemSusChem, 7 (8) 2289–2294 (2014)
Ma, S, Webster, DC, Jabeen, F, “Hard and Flexible, Degradable Thermosets from Renewable Bioresources with the Assistance of Water and Ethanol.” Macromolecules, 49 (10) 3780–3788 (2016)
Ma, S, Webster, DC, “Naturally Occurring Acids as Cross-Linkers to Yield VOC-Free, High-Performance, Fully Bio-based, Degradable Thermosets.” Macromolecules, 48 (19) 7127–7137 (2015)
Paramarta, A, Webster, DC, “The Exploration of Michael-Addition Reaction Chemistry to Create High Performance, Ambient Cure Thermoset Coatings Based on Soybean Oil.” Prog. Org. Coat., 108 59–67 (2017)
Paramarta, A, Webster, DC, “Bio-based High Performance Epoxy-Anhydride Thermosets for Structural Composites: The Effect of Composition Variables.” React. Funct. Polym., 105 140–149 (2016)
Webster DC, Yu AZ, “Biobased Highly Functional Oligomers and Thermosets Therefrom.” US Patent 9,765,233, 2017
Yu, AZ, Rahimi, A, Webster, DC, “High Performance Bio-based Thermosets from Dimethacrylated Epoxidized Sucrose Soyate (DMESS).” Eur. Polym. J., 99 202–211 (2018)
Yu, AZ, Sahouani, JM, Setien, RA, Webster, DC, “Effect of Nature and Extent of Functional Group Modification on Properties of Thermosets from Methacrylated Epoxidized Sucrose Soyate.” React. Funct. Polym., 128 29–39 (2018)
Pan, X, Sengupta, P, Webster, DC, “Novel Biobased Epoxy Compounds: Epoxidized Sucrose Esters of Fatty Acids.” Green Chem., 13 (4) 965–975 (2011)
Monono, EM, Webster, DC, Wiesenborn, DP, “Pilot Scale (10 kg) Production and Characterization of Epoxidized Sucrose Soyate.” Ind. Crops Prod., 74 987–997 (2015)
Monono, EM, Bahr, JA, Pryor, SW, Webster, DC, Wiesenborn, DP, “Optimizing Process Parameters of Epoxidized Sucrose Soyate Synthesis for Industrial Scale Production.” Org. Process Res. Dev., 19 (11) 1683–1692 (2015)
Samanta, S, Selvakumar, S, Bahr, J, Wickramaratne, DS, Sibi, M, Chisholm, BJ, “Synthesis and Characterization of Polyurethane Networks Derived from Soybean-Oil-Based Cyclic Carbonates and Bioderivable Diamines.” ACS Sustain. Chem. Eng., 4 (12) 6551–6561 (2016)
Lambeth, RH, Henderson, TJ, “Organocatalytic Synthesis of (Poly) Hydroxyurethanes from Cyclic Carbonates and Amines.” Polymer, 54 (21) 5568–5573 (2013)
Lombardo, VM, Dhulst, EA, Leitsch, EK, Wilmot, N, Heath, WH, Gies, AP, Miller, MD, Torkelson, JM, Scheidt, KA, “Cooperative Catalysis of Cyclic Carbonate Ring Opening: application Towards Non-Isocyanate Polyurethane Materials.” Eur. J. Org. Chem., 2015 (13) 2791–2795 (2015)
Cornille, A, Blain, M, Auvergne, R, Andrioletti, B, Boutevin, B, Caillol, S, “A Study of Cyclic Carbonate Aminolysis at Room Temperature: Effect of Cyclic Carbonate Structures and Solvents on Polyhydroxyurethane Synthesis.” Polym. Chem., 8 (3) 592–604 (2017)
Webster, DC, Crain, AL, “Synthesis and Applications of Cyclic Carbonate Functional Polymers in Thermosetting Coatings.” Prog. Org. Coat., 40 (1) 275–282 (2000)
Garipov, RM, Sysoev, VA, Mikheev, VV, Zagidullin, AI, Deberdeev, RY, Irzhak, VI, Berlin, AA, Reactivity of Cyclocarbonate Groups in Modified Epoxy–Amine Compositions, pp. 289–292. Springer, Berlin (2003)
Stockmayer, WH, “Molecular Distribution in Condensation Polymers.” J. Polym. Sci. Part A Polym. Chem., 9 (1) 69–71 (1952)
Stockmayer, WH, “Molecular Distribution in Condensation Polymers.” J. Polym. Sci. Part A Polym. Chem., 11 (5) 424 (1953)
Durand, D, Bruneau, CM, “Statistics of Random Macromolecular Networks, 2. Stepwise Polymerization of Polyfunctional Monomers Bearing A and B Coreactive Groups.” Macromol. Chem. Phys., 183 (4) 1021–1035 (1982)
Durand, D, Bruneau, C-M, “Average Functionalities of Macromolecules in Stepwise Polyfunctional Polymerization.” Polymer, 23 (1) 69–72 (1982)
Miller, DR, Macosko, CW, “Average Property Relations for Nonlinear Polymerization with Unequal Reactivity.” Macromolecules, 11 (4) 656–662 (1978)
Miller, DR, Valles, EM, Macosko, CW, “Calculation of Molecular Parameters for Stepwise Polyfunctional Polymerization.” Polym. Eng. Sci., 19 (4) 272–283 (1979)
Stafford, JW, “Multifunctional Polycondensation and Gelation: A Kinetic Approach.” J. Polym. Sci. Part A Polym. Chem., 19 (12) 3219–3236 (1981)
Nelson, TJ, Masaki, B, Morseth, Z, Webster, DC, “Highly Functional Biobased Polyols and Their Use in Melamine–Formaldehyde Coatings.” J. Coat. Technol. Res., 10 (6) 757–767 (2013)
Acknowledgments
This work was supported by the National Science Foundation EPSCoR Award under Grant No. IIA-1355466.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, A.Z., Setien, R.A., Sahouani, J.M. et al. Catalyzed non-isocyanate polyurethane (NIPU) coatings from bio-based poly(cyclic carbonates). J Coat Technol Res 16, 41–57 (2019). https://doi.org/10.1007/s11998-018-0135-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-018-0135-7