Abstract
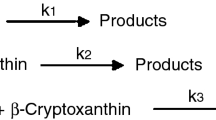
In order to give insight into β-carotene degradation mechanism during the storage of dried orange-fleshed sweet potato, and particularly into the role of isomers and norisoprenoids formation, multi-response kinetic modelling was applied. Determination of degradation compounds were carried out by HPLD-DAD and SPME-GC-MS as a function of time between 10 and 40 °C and at four water activities from 0.13 to 0.76. Kinetic modelling was developed assuming first-order reactions and by using mass balance. Eight compounds, namely, two isomers (9-cis- and 13-cis-β-carotene), two β-carotene epoxides (β-carotene 5,6 and 5,8 epoxide) and four volatile compounds (β-cyclocitral, β-ionone, 5,6-epoxy-β-ionone and dihydroactinidiolide), were integrated into two theoretical reaction schemes. The different models were discriminated according to goodness of fit to experimental data. This work showed that: (1) the formation of cis-isomers from β-carotene preceded oxidation, (2) β-cyclocitral arose directly from β-carotene scission while the other norisoprenoids resulted from β-carotene epoxide degradation, (3) cis-isomers were high reactive compounds. Temperature had a major influence on reaction rates k while water activities only impacted k at values under 0.51. Therefore, multi-response modelling is not only a tool to predict β-carotene degradation but a interesting way to select the appropriate degradation scheme based on the different options presented in literature.








Similar content being viewed by others
References
Achir, N., Pénicaud, C., Avallone, S., & Bohuon, P. (2011). Insight into β-carotene thermal degradation in oils with multiresponse modelling. Journal of the American Oil Chemist’s Society, 88(12), 2035–2045.
Bechoff, A. (2010c) Investigating carotenoid losses after drying and storage of orange-fleshed sweet potato. Natural Resources Institute, University of Greenwich.
Bechoff, A., Dhuique-Mayer, C., Dornier, M., Tomlins, K. I., Boulanger, R., Dufour, D., et al. (2010a). Relationship between the kinetics of [beta]-carotene degradation and formation of norisoprenoids in the storage of dried sweet potato chips. Food Chemistry, 121, 348–357.
Bechoff, A., Westby, A., Owori, C., Menya, G., Dhuique-Mayer, C., Dufour, D., et al. (2010b). Effect of drying and storage on the degradation of total carotenoids in orange-fleshed sweetpotato cultivars. Journal of the Science of Food and Agriculture, 90, 622–629.
Bechoff, A., Tomlins, K. I., Dhuique-Mayer, C., Dove, R., & Westby, A. (2011) On-farm evaluation of the impact of drying and subsequent storage on the carotenoid content of orange-fleshed sweet potato. International Journal of Food Science & Technology, 46, 52–60.
Benevides, C. M. J., Veloso, M. C. C., de Paula Pereira, P. A., & Andrade, J. B. (2011). A chemical study of β-carotene oxidation by ozone in an organic model system and the identification of the resulting products. Food Chemistry, 126(3), 927–934.
Bengtsson, A., Namutebi, A., Alminger, M. L., & Svanberg, U. (2008). Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. Journal of Food Composition and Analysis, 21(2), 134–143.
Bosser, A., Paplorey, E., & Belin, J. M. (1995). A simple way to (+/−)-dihydroactinidiolide from beta-ionone related to the enzymatic cooxidation of beta-carotene in aqueous-solution. Biotechnology Progress, 11(6), 689–692.
Chen, J. P., Tai, C. Y., & Chen, B. H. (2007). Effects of different drying treatments on the stability of carotenoids in Taiwanese mango (Mangifera indica L.). Food Chemistry, 100(3), 1005–1010.
Dhuique-Mayer, C., Tbatou, M., Carail, M., Caris-Veyrat, C., Dornier, M., & Amiot, M. J. (2007). Thermal degradation of antioxidant micronutrients in citrus juice: kinetics and newly formed compounds. Journal of Agricultural and Food Chemistry, 55(10), 4209–4216.
Gill, E. P., Murra, W., & Wright, M. H. (1981). Practical optimisation. New York: Academic Press.
Hiranvarachat, B., Suvarnakuta, P., & Devahastin, S. (2008). Isomerisation kinetics and antioxidant activities of [beta]-carotene in carrots undergoing different drying techniques and conditions. Food Chemistry, 107(4), 1538–1546.
Huet, S., Jolivet, E., & Messéan, A. (1992) La régression non-linéaire: méthodes et applications en biologie. Paris.
Koca, N., Burdurlu, H. S., & Karadeniz, F. (2007). Kinetics of colour changes in dehydrated carrots. Journal of Food Engineering, 78(2), 449–455.
Lan, C.-H. (2011). Stability of carotenoid extracts of Cucurbita maxima towards enzymatic cooxidation and aroma compound generation. IPCBEE, 7, 141–143.
Lavelli, V., Zanoni, B., & Zaniboni, A. (2007). Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chemistry, 104(4), 1705–1711.
Marx, M., Stuparic, M., Schieber, A., & Carle, R (2003) Effects of thermal processing on trans-cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. 83, 609–617.
Pénicaud, C., Achir, N., Dhuique-Mayer, C., Dornier, M., & Bohuon, P. (2011). Degradation of β-carotene during fruits and vegetables processing or storage: reaction mechanisms and kinetics aspects. FRUITS, 66(6), 417–440.
Pénicaud, C., Peyron, S., Gontard, N., & Guillard, V. (2012). Oxygen quantification methods and application to the determination of oxygen diffusion and solubility coefficients in food. Food Reviews International, 28(2), 113–145.
Rodriguez, E. B., & Rodriguez-Amaya, D. B. (2007). Formation of apocarotenals and epoxycarotenoids from [beta]-carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chemistry, 101(2), 563–572.
van Boekel, M. A. J. S. (2008). Kinetic modeling of reactions in foods. London: CRC.
Wache, Y., Bosser-DeRatuld, A., Lhuguenot, J. C., & Belin, J. M. (2003). Effect of cis/trans isomerism of beta-carotene on the ratios of volatile compounds produced during oxidative degradation. Journal of Agricultural and Food Chemistry, 51(7), 1984–1987.
Zeb, A. (2012). Oxidation and formation of oxidation products of β-carotene at boiling temperature. Chemistry and Physics of Lipids, 165(3), 277–281.
Zeb, A., & Murkovic, M. (2011). Determination of thermal oxidation and oxidation products of β-carotene in corn oil triacylglycerols. Food Research International, 50(2), 534–544.
Zhang, P., & Omaye, S. T. (2001). [beta]-Carotene: interactions with [alpha]-tocopherol and ascorbic acid in microsomal lipid peroxidation. The Journal of Nutritional Biochemistry, 12(1), 38–45.
Acknowledgments
The authors thank HarvestPlus and DESI-funding from CIRAD for supporting the PhD thesis that generated the data that were used for this mathematical modelling. The views expressed are however those of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achir, N., Pénicaud, C., Bechoff, A. et al. Use of Multi-response Modelling to Investigate Mechanisms of β-Carotene Degradation in Dried Orange-Fleshed Sweet Potato During Storage: from Carotenoids to Aroma Compounds. Food Bioprocess Technol 7, 1656–1669 (2014). https://doi.org/10.1007/s11947-013-1229-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1229-y