Abstract
Purpose of the review
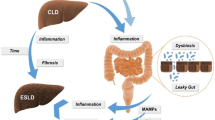
Liver disease, colon cancer, and the gut microbiome are intimately interrelated; however, the connections linking liver disease and colorectal neoplasia via the gut microbiota remain poorly understood and rarely addressed in a single space. The goal of this review is to take a broad perspective on the clinical problem of colorectal neoplasia in the liver disease population, recognize the significance of the clinical study findings, and delve into the evidence supporting putative molecular mechanisms connecting dysbiosis in the progression of liver disease to the development of colorectal neoplasia.
Recent findings
Clinical studies have recently reported increased risk of colorectal neoplasia in patients with fatty liver disease, and risk increases with liver disease severity. Concurrently, the evolution of -omics technology has shown dysregulation of the gut microbial community, termed dysbiosis, in the progression of liver disease. Specific microbes enriched in the gut flora of liver disease patients have been linked to colon cancer and adenomatous precursor lesions.
Summary
The gut microbiome of liver disease patients generates a pro-neoplastic environment, mediated via altered bile acid signaling and a dysregulated inflammatory response that suppresses immune surveillance. Research focused on the mechanisms linking liver disease to colorectal neoplasia via the gut microbiome is needed to help us prepare for the rising tide of colon cancer in young patients with an increasing prevalence of liver disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
ABELS JC, et al. Metabolic studies in patients with cancer of the gastrointestinal tract. II. Hepatic dysfunction. Ann Intern Med. 1942;16(2):221–40.
Parker RG, Kendall EJ. The liver in ulcerative colitis. Br Med J. 1954;2(4845):1030–2.
Smith MP, Loe RH. Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am J Surg. 1965;110:239–46.
Mistilis SP. Pericholangitis and ulcerative colitis. I. Pathology, etiology, and pathogenesis. Ann Intern Med. 1965;63:1–16.
Sivak MV Jr, Farmer RG, Lalli F. Sclerosing cholangitis: its increasing frequency of recognition and association with inflammatory bowel disease. J Clin Gastroenterol. 1981;3(3):261–6.
D’Haens GR, Lashner BA, Hanauer SB. Pericholangitis and sclerosing cholangitis are risk factors for dysplasia and cancer in ulcerative colitis. Am J Gastroenterol. 1993;88(8):1174–8.
Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56(1):48–54.
Lindor KD, Kowdley KV, Harrison ME. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110(5):646–59 quiz 660.
Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322–33.
Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis. J Gastrointest Oncol. 2014;5(6):440–6.
Wong VW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829–36.
Siegel RL, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8). https://doi.org/10.1093/jnci/djw322.
Asrani SK, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171.
Komaki Y, et al. Risk of colorectal cancer in chronic liver diseases: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86(1):93–104.e5.
•• Llorente C, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun. 2017;8(1):837 Translational analysis of the effect of acid suppression on gut microbiota and the its relationship to the natural history of alcoholic liver disease. This paper linked a clinical exposure to microbiota shift and identified a specific organism responsible for accelerating liver disease.
Jia W, Xie G. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–28.
Jaensson-Gyllenback E, et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011;4(4):438–47.
Czarnewski P, et al. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients. 2017;9(1):e68.
Chalasani N, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357.
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95.
Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47(2):239–44.
Mantovani A, Dauriz M, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metabolism. 2018;87:1–12.
Lee T, Yun KE, Chang Y, Ryu S, Park DI, Choi K, et al. Risk of colorectal neoplasia according to fatty liver severity and presence of gall bladder polyps. Dig Dis Sci. 2016;61(1):317–24.
Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS, Jung SH, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther. 2017;45(2):345–53.
Bhatt BD, et al. Increased risk of colorectal polyps in patients with non-alcoholic fatty liver disease undergoing liver transplant evaluation. J Gastrointest Oncol. 2015;6(5):459–68.
Bardou M, Montembault S, Giraud V, Balian A, Borotto E, Houdayer C, et al. Excessive alcohol consumption favours high risk polyp or colorectal cancer occurrence among patients with adenomas: a case control study. Gut. 2002;50(1):38–42.
Song YK, Park YS, Seon CS, Lim HJ, Son BK, Ahn SB, et al. Alcohol drinking increased the risk of advanced colorectal adenomas. Intest Res. 2015;13(1):74–9.
Kim SH, Kim JW, Lee KL, Lee S, Koh SJ, Jeong JB, et al. Hepatitis B virus infection is independently associated with advanced colorectal adenoma. Am J Med Sci. 2018;356(2):141–6.
Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Collier J. Liver cirrhosis, other liver diseases, pancreatitis and subsequent cancer: record linkage study. Eur J Gastroenterol Hepatol. 2008;20(5):384–92.
Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117(6):1163–71.
Singh S, Khanna S, Pardi DS, Loftus EV Jr, Talwalkar JA. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19(8):1631–8.
Singh S, Varayil JE, Loftus EV Jr, Talwalkar JA. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 2013;19(12):1361–9.
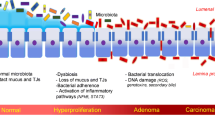
Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–8.
Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372(15):1441–52.
Saltzman ET, Palacios T, Thomsen M, Vitetta L. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front Microbiol. 2018;9:61.
Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, et al. Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis. 2018;67(6):869–77.
Wang J, Wang Y, Zhang X, Liu J, Zhang Q, Zhao Y, et al. Gut microbial dysbiosis is associated with altered hepatic functions and serum metabolites in chronic hepatitis B patients. Front Microbiol. 2017;8:2222.
Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39(5):763–75.
•• Jiang W, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096 Critical paper that examined the compositional changes in gut microbiota of NAFLD patients. They also assessed changes in immune signaling and T lymphocyte populations in patient biopsies together, showing the microbial populations were linked to altered immune activity in the gut mucosa.
Chang C, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol. 2019; 103(3):1393–1404.
• Kostic AD, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15 One of the first groups to identify Fusobacterium nucleatum in colorectal adenocarcinomas, this follow on paper addressed the mechanistic link and molecular pathway whereby the bacterium supports the development of neoplasia.
Yan G, et al. A RIPK3-PGE2 circuit mediates myeloid-derived suppressor cell-potentiated colorectal carcinogenesis. Cancer Res. 2018;78(19):5586–99.
Mori G, Rampelli S, Orena BS, Rengucci C, de Maio G, Barbieri G, et al. Shifts of faecal microbiota during sporadic colorectal carcinogenesis. Sci Rep. 2018;8(1):10329.
Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4(1):69.
Rezasoltani S, Asadzadeh Aghdaei H, Dabiri H, Akhavan Sepahi A, Modarressi MH, Nazemalhosseini Mojarad E. The association between fecal microbiota and different types of colorectal polyp as precursors of colorectal cancer. Microb Pathog. 2018;124:244–9.
Park CH, Han DS, Oh YH, Lee AR, Lee YR, Eun CS. Role of fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep. 2016;6:25271.
Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7(6):e39743.
Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411.
Mira-Pascual L, Cabrera-Rubio R, Ocon S, Costales P, Parra A, Suarez A, et al. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J Gastroenterol. 2015;50(2):167–79.
Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. Role of oral microbiome on oral cancers, a review. Biomed Pharmacother. 2016;84:552–8.
Strauss J, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971–8.
Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306.
Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8.
Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72.
Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017:2(19):94416.
Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62(4):1260–71.
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65(12):2035–44.
Abdulamir AS, Hafidh RR, Abu Bakar F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30:11.
Sun J, Kato I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016;3(2):130–43.
Boursi B, Haynes K, Mamtani R, Yang YX. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015;24(5):534–42.
Kaur K, Saxena A, Debnath I, O’Brien JL, Ajami NJ, Auchtung TA, et al. Antibiotic-mediated bacteriome depletion in Apc(Min/+) mice is associated with reduction in mucus-producing goblet cells and increased colorectal cancer progression. Cancer Med. 2018;7(5):2003–12.
Enblad M, Birgisson H, Ekbom A, Sandin F, Graf W. Increased incidence of bowel cancer after non-surgical treatment of appendicitis. Eur J Surg Oncol. 2017;43(11):2067–75.
Cao Y, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–8.
Theochari NA, Stefanopoulos A, Mylonas KS, Economopoulos KP. Antibiotics exposure and risk of inflammatory bowel disease: a systematic review. Scand J Gastroenterol. 2018;53(1):1–7.
Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109(11):1728–38.
Starkel P, Schnabl B. Bidirectional communication between liver and gut during alcoholic liver disease. Semin Liver Dis. 2016;36(4):331–9.
Brenner DA, Paik YH, Schnabl B. Role of gut microbiota in liver disease. J Clin Gastroenterol. 2015;49(Suppl 1):S25–7.
Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34–40.
Wei X, et al. Community-metabolome correlations of gut microbiota from Child-Turcotte-Pugh of A and B patients. Front Microbiol. 2016;7:1856.
Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, et al. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. 2013;13:175.
Kosmalski M, Mokros Ł, Kuna P, Witusik A, Pietras T. Changes in the immune system - the key to diagnostics and therapy of patients with non-alcoholic fatty liver disease. Cent Eur J Immunol. 2018;43(2):231–9.
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–7.
Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85(8):863–71.
Ridlon JM, Wolf PG, Gaskins HR. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes. 2016;7(3):201–15.
Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50(8):1509–20.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101.
Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31a(7–8):1067–70.
• Schulz MD, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–12 This paper used a high-fat diet mouse model to separate the relative contributions of obesity and its complications from dysbiosis in intestinal oncogenesis. Importantly, they demonstrate high-fat diet-induced shifts in gut microbiota that potentiated cancer in the absence of obesity and fatty liver, anchoring the microbiota-carcinoma hypothesis.
Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140(11):2545–56.
Calmus Y, Poupon R. Shaping macrophages function and innate immunity by bile acids: mechanisms and implication in cholestatic liver diseases. Clin Res Hepatol Gastroenterol. 2014;38(5):550–6.
Sun L, Beggs K, Borude P, Edwards G, Bhushan B, Walesky C, et al. Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G91–g104.
Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306(11):G929–37.
Chazouillères O. Primary sclerosing cholangitis and bile acids. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S21–5.
Caussy C, et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. 2019; 49(2):183–193.
Wang X, Xie G, Zhao A, Zheng X, Huang F, Wang Y, et al. Serum bile acids are associated with pathological progression of hepatitis B-induced cirrhosis. J Proteome Res. 2016;15(4):1126–34.
Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16(9):885–96.
Vernia P, Gnaedinger A, Hauck W, Breuer RI. Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci. 1988;33(11):1353–8.
Wong JM, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43.
Chen J, Vitetta L. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Cancer. 2018;17(3):e541–4.
Li Q, Cao L, Tian Y, Zhang P, Ding C, Lu W, et al. Butyrate suppresses the proliferation of colorectal cancer cells via targeting pyruvate kinase M2 and metabolic reprogramming. Mol Cell Proteomics. 2018;17(8):1531–45.
Xu Z, et al. Sodium butyrate inhibits colorectal cancer cell migration by downregulating Bmi-1 through enhanced miR-200c expression. Mol Nutr Food Res. 2018;62(6):e1700844.
Loomba R, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25(5):1054–1062.e5.
Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019. https://doi.org/10.1111/liv.14106.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Gleeson declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is part of the Topical Collection on Colon
Rights and permissions
About this article
Cite this article
Gleeson, M.W. Interplay of Liver Disease and Gut Microbiota in the Development of Colorectal Neoplasia. Curr Treat Options Gastro 17, 378–393 (2019). https://doi.org/10.1007/s11938-019-00241-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00241-6