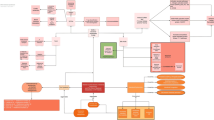
Opinion statement
Heart failure is the leading cause of mortality and morbidity in the world today. While there have been major advances in our understanding of the pathophysiology of heart failure over the past decades, disease progression remains inevitable in the majority of patients and effective therapies to prevent heart failure are still lacking. Research has turned to better understand the gut microbiome because alterations in their ecosystems have been associated with various downstream chronic conditions including cardiovascular diseases. The gut microbiome is complex and diverse in nature, making it difficult to generalize to specific populations or individual patients. Nevertheless, current evidence has found links between heart failure and alterations in microbial composition and function, since heart failure has long been associated with impaired intestinal barrier function and bacterial translocation leading to inflammatory and immune responses. Recent studies have also shed light on the contributions of gut microbiota-derived metabolites from dietary nutrients that can promote adverse effects in the setting of cardiorenal diseases. In this review, we will discuss the role of gut microbiome in the setting of heart failure and potential interventional approaches that may potentially lower the risk of disease progression in heart failure.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Green ED, Watson JD, Collins FS. Human Genome Project: twenty-five years of big biology. Nature. 2015;526(7571):29–31.
Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80.
Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol. 2016;35:8–15.
Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–60.
Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124(10):4204–11.
Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–4.
Yadav D, Ghosh TS, Mande SS. Global investigation of composition and interaction networks in gut microbiomes of individuals belonging to diverse geographies and age-groups. Gut Pathog. 2016;8:17.
Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84.
•• Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. Seminal paper describing the contributory role of trimethylamine N-oxide in atherogenesis and the obligatory participation of gut microbiome.
Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146(6):1564–72.
Lawson-Yuen A, Levy HL. The use of betaine in the treatment of elevated homocysteine. Mol Genet Metab. 2006;88(3):201–7.
Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31(11-12):1317–23.
Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel). 2016;8(11):326.
Senthong V, Li XS, Hudec T, et al. Plasma trimethylamine N-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67(22):2620–8.
Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH. Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS One. 2015;10(12):e0143731.
Missailidis C, Hallqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738.
Shafi T, Powe NR, Meyer TW, et al. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol. 2016;28(1):321–31.
Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–13.
Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55.
•• Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–42. Prospective demonstration of endotoxin and immune activation in heart failure.
• Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16):1561–9. Key demonstration of the contributions of altered intestinal function in heart failure.
Sharma R, Bolger AP, Rauchhaus M, et al. Cellular endotoxin desensitization in patients with severe chronic heart failure. Eur J Heart Fail. 2005;7(5):865–8.
Sharma R, von Haehling S, Rauchhaus M, et al. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur J Heart Fail. 2005;7(4):479–84.
Sandek A, Bjarnason I, Volk HD, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157(1):80–5.
• Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5(5):609–14. Early work demonstrating the presence of bacterial endotoxin and inflammatory cytokines in acute heart failure.
von Haehling S, Genth-Zotz S, Bolger AP, et al. Effect of noradrenaline and isoproterenol on lipopolysaccharide-induced tumor necrosis factor-alpha production in whole blood from patients with chronic heart failure and the role of beta-adrenergic receptors. Am J Cardiol. 2005;95(7):885–9.
Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220–7.
Phillips Campbell RB, Duffourc MM, Schoborg RV, et al. Aberrant fecal flora observed in guinea pigs with pressure overload is mitigated in animals receiving vagus nerve stimulation therapy. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G754–62.
• Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–14. First demonstration of association between elevated TMAO and prognosis in heart failure.
Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21(2):91–6.
Troseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277(6):717–26.
Gabe SM, Bjarnason I, Tolou-Ghamari Z, et al. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology. 1998;115(1):67–74.
Dickson RP, Erb-Downward JR, Freeman CM, et al. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One. 2014;9(5):e97214.
Kitai T, Kirsop J, Tang WH. Exploring the microbiome in heart failure. Curr Heart Fail Rep. 2016;13(2):103–9.
• Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9(1):e002314. Animal studies demonstrating the contribution of dietary-induced TMAO production and cardiac remodeling in mouse model.
Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90.
De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2015;28:gutjnl-2015.
Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
Miller MJ, Bostwick BL, Kennedy AD, et al. Chronic oral L-carnitine supplementation drives marked plasma TMAO elevations in patients with organic acidemias despite dietary meat restrictions. JIMD Rep. 2016;30:39–44.
Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179:348–50.
• Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7(3):491–9. Probiotics intervention with signals of attenuating cardiac remodeling in a rat heart failure model.
Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25(8):1919–30.
Fujii H, Nishijima F, Goto S, et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009;24(7):2089–95.
Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60.
Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290(9):5647–60.
Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37.
Tang WH, Wang Z, Li XS, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2016;63(1):297–306.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Wilson Tang is supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827). Dr. Tang is a section editor for Current Treatment Options in Cardiovascular Medicine.
Allyson Zabell declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
Zabell, A., Tang, W.H.W. Targeting the Microbiome in Heart Failure. Curr Treat Options Cardio Med 19, 27 (2017). https://doi.org/10.1007/s11936-017-0528-4
Published:
DOI: https://doi.org/10.1007/s11936-017-0528-4