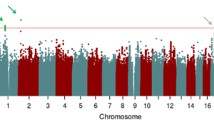
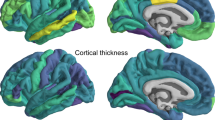
Abstract
Purpose of Review
We review recent progress in uncovering the complex genetic architecture of cognition, arising primarily from genome-wide association studies (GWAS). We explore the genetic correlations between cognitive performance and neuropsychiatric disorders, the genetic and environmental factors associated with age-related cognitive decline, and speculate about the future role of genomics in the understanding of cognitive processes.
Recent Findings
Improvements in genomic methods, and the increasing availability of large datasets via consortia cooperation, have led to a greater understanding of the role played by common and rare variants in the genomics of cognition, the highly polygenic basis of cognitive function and dysfunction, and the multiple biological processes involved.
Summary
Recent research has aided in our understanding of the complex biological nature of genomics of cognition. Further development of data banks and techniques to analyze this data hold significant promise for understanding cognitive ability, and for treating cognitively related disability.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2014;20:98. https://doi.org/10.1038/mp.2014.105.
Plomin R, Spinath F. Intelligence: genetics, genes, and genomics. J Pers Soc Psychol. 2004;86:112–29. https://doi.org/10.1037/0022-3514.86.1.112.
Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 4th ed. New York: Worth Publishers; 2001.
Briley DA, Tucker-Drob EM. Explaining the increasing heritability of cognitive ability across development: a meta-analysis of longitudinal twin and adoption studies. Psychol Sci. 2013;24(9):1704–13. https://doi.org/10.1177/0956797613478618.
Ramus F. Genes, brain, and cognition: a roadmap for the cognitive scientist. Cognition. 2006;101(2):247–69. https://doi.org/10.1016/j.cognition.2006.04.003.
• Plomin R, von Stumm S. The new genetics of intelligence. Nat Rev Genet. 2018;19:148. https://doi.org/10.1038/nrg.2017.104 This recent review article discusses the benefits of polygenic scores in intelligence research.
•• Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50(8):1112–21. https://doi.org/10.1038/s41588-018-0147-3 This study is the largest GWAS study to date on educational attainment using both public and commerically available data and highlights the role of genes involved in the prenatal brain as well post natal development.
• Lam M, Hill WD, Trampush JW, Yu J, Knowles E, Davies G, et al. Pleiotropic meta-analysis of cognition, education, and schizophrenia differentiates roles of early neurodevelopmental and adult synaptic pathways. bioRxiv. 2019;105:334. https://doi.org/10.1101/519967 This paper examines the pleiotropic nature of GWAS findings on intelligence and psychiatric disorders.
Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–11. https://doi.org/10.1038/nrn2793.
Johnson W, Nijenhuis J, Bouchard TJ. Still just 1 g: consistent results from five test batteries. Intelligence. 2008;36(1):81–95. https://doi.org/10.1016/j.intell.2007.06.001.
Warne RT, Burningham C. Spearman’s g found in 31 non-Western nations: strong evidence that g is a universal phenomenon. Psychol Bull. 2019;145(3):237–72. https://doi.org/10.1037/bul0000184.
Hedge C, Powell G, Sumner P. The reliability paradox: why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods. 2018;50(3):1166–86. https://doi.org/10.3758/s13428-017-0935-1.
De Schryver M, Hughes S, Rosseel Y, De Houwer J. Unreliable yet still replicable: a comment on LeBel and Paunonen (2011). Front Psychol. 2016;6(2039). https://doi.org/10.3389/fpsyg.2015.02039.
Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539–42. https://doi.org/10.1038/nature17671.
Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. medRxiv. 2019;19002204. https://doi.org/10.1101/19002204.
Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35(1):13–21. https://doi.org/10.1016/j.intell.2006.02.001.
Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 2017;49(7):1107–12. https://doi.org/10.1038/ng.3869.
Trampush JW, Yang ML, Yu J, Knowles E, Davies G, Liewald DC, et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry. 2017;22(3):336–45. https://doi.org/10.1038/mp.2016.244.
Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53949). Mol Psychiatry. 2015;20(2):183–92. https://doi.org/10.1038/mp.2014.188.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779.
Hill WD, Davies G, Harris SE, Hagenaars SP, Liewald DC, Penke L, et al. Molecular genetic aetiology of general cognitive function is enriched in evolutionarily conserved regions. Transl Psychiatry. 2016;6(12):e980. https://doi.org/10.1038/tp.2016.246.
Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, et al. Genome-wide association study of cognitive functions and educational attainment in UK biobank (N=112 151). Mol Psychiatry. 2016;21(6):758–67. https://doi.org/10.1038/mp.2016.45.
•• Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. 2018;9(1):2098. https://doi.org/10.1038/s41467-018-04362-x This study demonstrates the power of sample size to GWAS findings by the addition of the UK Biobank data. It confirms previous findings and identifies new loci associated with neuronial communication.
•• Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912–9. https://doi.org/10.1038/s41588-018-0152-6 Again using the UK Biobank and other data, this study had identified the largest number of association snps for IQ to date, and reports a bio-informatic analysis of these findings.
Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6(6):1–20. https://doi.org/10.1371/journal.pgen.1000993.
Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–37. https://doi.org/10.1038/s41588-017-0009-4.
• Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, AM MI, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-017-0001-5 Hill et al. demonstrate the additive effects of multi-trait anlaysis and its utility to generate further findings.
Lee C, Scherer SW. The clinical context of copy number variation in the human genome. Expert Rev Mol Med. 2010;12:e8. https://doi.org/10.1017/S1462399410001390.
Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7(2):85–97. https://doi.org/10.1038/nrg1767.
Kendall KM, Rees E, Escott-Price V, Einon M, Thomas R, Hewitt J, et al. Cognitive performance among carriers of pathogenic copy number variants: analysis of 152,000 UK Biobank subjects. Biol Psychiatry. 2017;82(2):103–10. https://doi.org/10.1016/j.biopsych.2016.08.014.
Nowakowska B. Clinical interpretation of copy number variants in the human genome. J Appl Genet. 2017;58(4):449–57. https://doi.org/10.1007/s13353-017-0407-4.
Huddleston J, Chaisson MJP, Steinberg KM, Warren W, Hoekzema K, Gordon D, et al. Discovery and genotyping of structural variation from long-read haploid genome sequence data. Genome Res. 2017;27(5):677–85.
Chiang C, Scott AJ, Davis JR, Tsang EK, Li X, Kim Y, et al. The impact of structural variation on human gene expression. Nat Genet. 2017;49:692–9. https://doi.org/10.1038/ng.3834.
• Huguet G, Schramm C, Douard E, Jiang L, Labbe A, Tihy F, et al. Measuring and estimating the effect sizes of copy number variants on general intelligence in community-based samples. JAMA Psychiatry. 2018;75(5):447–57. https://doi.org/10.1001/jamapsychiatry.2018.0039 This study presents a framework for examining the effects of copy number variants on general cognition.
• Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2013;505:361. https://doi.org/10.1038/nature12818 The study of population isolates can highlight the role of CNVs in cognition.
Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–39. https://doi.org/10.1038/mp.2010.4.
Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, et al. Cohort profile: the Saguenay Youth Study (SYS). Int J Epidemiol. 2017;46(2):e19. https://doi.org/10.1093/ije/dyw023.
Kendall KM, Bracher-Smith M, Fitzpatrick H, Lynham A, Rees E, Escott-Price V, et al. Cognitive performance and functional outcomes of carriers of pathogenic copy number variants: analysis of the UK Biobank. Br J Psychiatry. 2019;214(5):297–304. https://doi.org/10.1192/bjp.2018.301.
Warland A, Kendall KM, Rees E, Kirov G, Caseras X. Schizophrenia-associated genomic copy number variants and subcortical brain volumes in the UK Biobank. Mol Psychiatry. 2019:1–9. https://doi.org/10.1038/s41380-019-0355-y.
•• Ganna A, Genovese G, Howrigan DP, Byrnes A, Kurki MI, Zekavat SM et al. Ultra-rare disruptive and damaging mutations influence educational attainment in the general population. Nat Neurosci. 2016;19:1563. doi:https://doi.org/10.1038/nn.4404 Important paper highlighting the role of rare mutations in intelligence using EA as a proxy measure.
• Hill WD, Arslan RC, Xia C, Luciano M, Amador C, Navarro P, et al. Genomic analysis of family data reveals additional genetic effects on intelligence and personality. Mol Psychiatry. 2018;23(12):2347–62. https://doi.org/10.1038/s41380-017-0005-1 This study shows how using family based data can resolve some of the missing hertiability in cognition.
Smith BH, Campbell H, Blackwood D, Connell J, Connor M, Deary IJ, et al. Generation Scotland: the Scottish Family Health Study; a new resource for researching genes and heritability. BMC Med Genet. 2006;7(1):74. https://doi.org/10.1186/1471-2350-7-74.
Zaitlen N, Kraft P, Patterson N, Pasaniuc B, Bhatia G, Pollack S, et al. Using extended genealogy to estimate components of heritability for 23 quantitative and dichotomous traits. PLoS Genet. 2013;9(5):e1003520. https://doi.org/10.1371/journal.pgen.1003520.
Xia C, Amador C, Huffman J, Trochet H, Campbell A, Porteous D, et al. Pedigree- and SNP-associated genetics and recent environment are the major contributors to anthropometric and cardiometabolic trait variation. PLoS Genet. 2016;12(2):e1005804. https://doi.org/10.1371/journal.pgen.1005804.
Shafee R, Nanda P, Padmanabhan JL, Tandon N, Alliey-Rodriguez N, Kalapurakkel S, et al. Polygenic risk for schizophrenia and measured domains of cognition in individuals with psychosis and controls. Transl Psychiatry. 2018;8(1):78. https://doi.org/10.1038/s41398-018-0124-8.
• Hasan A, Afzal M. Gene and environment interplay in cognition: Evidence from twin and molecular studies, future directions and suggestions for effective candidate gene x environment (cGxE) research. Mult Scler Relat Disord. 2019;33:121–30. https://doi.org/10.1016/j.msard.2019.05.005 This article reviews the interplay between the enviroment and cognition and explores the need to examine these effects in future studies.
Cheesman R, Hunjan A, Coleman JRI, Ahmadzadeh Y, Plomin R, McAdams TA, et al. Comparison of adopted and non-adopted individuals reveals gene-environment interplay for education in the UK Biobank. bioRxiv. 2019:707695. https://doi.org/10.1101/707695.
Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE, et al. The nature of nurture: effects of parental genotypes. Science. 2018;359(6374):424–8. https://doi.org/10.1126/science.aan6877.
Selzam S, Ritchie SJ, Pingault J-B, Reynolds CA, O’Reilly PF, Plomin R. Comparing within- and between-family polygenic score prediction. bioRxiv. 2019:605006. https://doi.org/10.1101/605006.
Toulopoulou T, Goldberg T, Rebollo Mesa I, Picchioni M, Rijsdijk F, Stahl D, et al. Impaired intellect and memory a missing link between genetic risk and schizophrenia? Arch Gen Psychiatry. 2010;67:905–13. https://doi.org/10.1001/archgenpsychiatry.2010.99.
Fowler D, Hodgekins J, Garety P, Freeman D, Kuipers E, Dunn G, et al. Negative cognition, depressed mood, and paranoia: a longitudinal pathway analysis using structural equation modeling. Schizophr Bull. 2012;38(5):1063–73. https://doi.org/10.1093/schbul/sbr019.
Blokland GAM, Del Re EC, Mesholam-Gately RI, Jovicich J, Trampush JW, Keshavan MS, et al. The Genetics of Endophenotypes of Neurofunction to Understand Schizophrenia (GENUS) consortium: a collaborative cognitive and neuroimaging genetics project. Schizophr Res. 2018;195:306–17. https://doi.org/10.1016/j.schres.2017.09.024.
Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19(2):168–74. https://doi.org/10.1038/mp.2013.166.
Hubbard L, Tansey KE, Rai D, Jones P, Ripke S, Chambert KD, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2015;42(3):832–42. https://doi.org/10.1093/schbul/sbv168.
Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DC, Ritchie SJ, et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry. 2016;21(11):1624–32. https://doi.org/10.1038/mp.2015.225.
Fanous AH, Zhou B, Aggen SH, Bergen SE, Amdur RL, Duan J, et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatry. 2012;169(12):1309–17. https://doi.org/10.1176/appi.ajp.2012.12020218.
•• Richards AL, Pardiñas AF, Frizzati A, Tansey KE, Lynham AJ, Holmans P, et al. The Relationship Between Polygenic Risk Scores and Cognition in Schizophrenia. Schizophr Bull. 2019. https://doi.org/10.1093/schbul/sbz061 This study provides a current estimate of the genetic correlation between cognitive function and schizophrenia susceptibility.
Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. 2016;77(Suppl 2):8–11. https://doi.org/10.4088/jcp.14074su1c.02.
World Health O. World report on ageing and health. Geneva: World Health Organization; 2015.
Andrews SJ, Das D, Cherbuin N, Anstey KJ, Easteal S. Association of genetic risk factors with cognitive decline: the PATH through life project. Neurobiol Aging. 2016;41:150–8. https://doi.org/10.1016/j.neurobiolaging.2016.02.016.
Tucker-Drob EM, Brandmaier AM, Lindenberger U. Coupled cognitive changes in adulthood: a meta-analysis. Psychol Bull. 2019. https://doi.org/10.1037/bul0000179.
•• Cabeza R, Albert M, Belleville S, FIM C, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701–10. https://doi.org/10.1038/s41583-018-0068-2 This article presents a clear theortitical model to explain the factors involved in cognitive decline.
Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018. https://doi.org/10.1016/j.jalz.2018.07.219.
• Ritchie SJ, Hill WD, Marioni RE, Davies G, Hagenaars SP, Harris SE, et al. Polygenic predictors of age-related decline in cognitive ability. Mol Psychiatry. 2019. https://doi.org/10.1038/s41380-019-0372-x This study explores the use of PGS in understanding the genetics of cognitive decline and highlights the need for further work.
Deary IJ, Yang J, Davies G, Harris SE, Tenesa A, Liewald D, et al. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482(7384):212–5. https://doi.org/10.1038/nature10781.
Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182–93. https://doi.org/10.7326/0003-4819-153-3-201008030-00258.
•• Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–99.e5. https://doi.org/10.1016/j.stem.2018.03.015 This research explores new frontiers in the understanding of cognitive variance in aging and proposes novel concepts involving neurogenesis.
Van Hout CV, Tachmazidou I, Backman JD, Hoffman JX, Ye B, Pandey AK, et al. Whole exome sequencing and characterization of coding variation in 49,960 individuals in the UK Biobank. bioRxiv. 2019;572347. https://doi.org/10.1101/572347.
• Eichler EE. Genetic variation, comparative genomics, and the diagnosis of disease. N Engl J Med. 2019;381(1):64–74. https://doi.org/10.1056/NEJMra1809315 Very good review of the challenges facing genetic studies into the future.
McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–83. https://doi.org/10.1038/ng.3643.
McClellan JM, Lehner T, King M-C. Gene discovery for complex traits: lessons from Africa. Cell. 2017;171(2):261–4. https://doi.org/10.1016/j.cell.2017.09.037.
Rietveld CA, Esko T, Davies G, Pers TH, Turley P, Benyamin B, et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc Natl Acad Sci. 2014;111(38):13790–4. https://doi.org/10.1073/pnas.1404623111.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. https://doi.org/10.1038/s41588-018-0269-7.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431–44. https://doi.org/10.1038/s41588-019-0344-8.
Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Pavlides JMW et al. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705–15.e16. doi: https://doi.org/10.1016/j.cell.2018.05.046.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668–81. https://doi.org/10.1038/s41588-018-0090-3.
Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381–9. https://doi.org/10.1038/s41588-018-0059-2.
Watanabe K, Stringer S, Frei O, Umićević Mirkov M, de Leeuw C, Polderman TJC, et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet. 2019;51(9):1339–48. https://doi.org/10.1038/s41588-019-0481-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Article is part of the Topical Collection on Genetic Disorders
Rights and permissions
About this article
Cite this article
Fitzgerald, J., Morris, D.W. & Donohoe, G. Cognitive Genomics: Recent Advances and Current Challenges. Curr Psychiatry Rep 22, 2 (2020). https://doi.org/10.1007/s11920-019-1125-x
Published:
DOI: https://doi.org/10.1007/s11920-019-1125-x