Abstract
Purpose of Review
Type 1 diabetes (T1D) occurs when there is destruction of beta cells within the islets of Langerhans in the pancreas due to autoimmunity. It is considered a complex disease, and different complications can surface and worsen the condition if T1D is not managed well. Since it is an incurable disease, numerous treatments and therapies have been postulated in order to control T1D by balancing hyperglycemia control while minimizing hypoglycemic episodes. The purpose of this review is to primarily look into the current state of the available immunological therapies and their advantages for the treatment of T1D.
Recent Findings
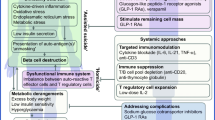
Over the years, immunological therapy has become the center of attraction to treat T1D. Immunomodulatory approaches on non-antigens involving agents such as cyclosporine A, mycophenolate mofetil, anti-CD20, cytotoxic T cells, anti-TNF, anti-CD3, and anti-thymocyte globulin as well as immunomodulative approaches on antigens such as insulin, glutamic acid decarboxylase, and heat shock protein 60 have been studied. Aside from these two approaches, studies and trials have also been conducted on regulatory T cells, dendritic cells, interleukin 2, interleukin 4, M2 macrophages, and rapamycin/interleukin 2 combination therapy to test their effects on patients with T1D. Many of these agents have successfully suppressed T1D in non-obese diabetic (NOD) mice and in human trials. However, some have shown negative results.
Summary
To date, the insights into the management of the immune system have been increasing rapidly to search for potential therapies and treatments for T1D. Nevertheless, some of the challenges are still inevitable. A lot of work and effort need to be put into the investigation on T1D through immunological therapy, particularly to reduce complications to improve and enhance clinical outcomes.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62–9. https://doi.org/10.2337/dc10-S062.
Gupta G, de Jesus Andreoli Pinto T, Chellappan DK, Mishra A, Malipeddi H, Dua K. A clinical update on metformin and lung cancer in diabetic patients. Panminerva Med. 2018;60(2):70–5. https://doi.org/10.23736/s0031-0808.18.03394-3.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S9. https://doi.org/10.2337/dc10-S062.
Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(11). doi:https://doi.org/10.1101/cshperspect.a007641.
Eisenbarth GS. Update in type 1 diabetes. J Clin Endocrinol Metab. 2007;92(7):2403–7. https://doi.org/10.1210/jc.2007-0339.
Morales J, Schneider D. Hypoglycemia. Am J Med. 2014;127(10 Suppl):S17–24. https://doi.org/10.1016/j.amjmed.2014.07.004.
Michels A, Zhang L, Khadra A, Kushner JA, Redondo MJ, Pietropaolo M. Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention. Pediatr Diabetes. 2015;16(7):465–84.
Rigby MR, Ehlers MR. Targeted immune interventions for type 1 diabetes: not as easy as it looks! Curr Opin Endocrinol Diabetes Obes. 2014;21(4):271–8. https://doi.org/10.1097/med.0000000000000075.
Weigmann B, Franke RK, Daniel C. Immunotherapy in autoimmune type 1 diabetes. Rev Diabet Stud. 2012;9(2–3):68–81. https://doi.org/10.1900/rds.2012.9.68.
Durnian JM, Stewart RM, Tatham R, Batterbury M, Kaye SB. Cyclosporin-A associated malignancy. Clin Ophthalmol. 2007;1(4):421–30.
Chatenoud L, Warncke K, Ziegler AG. Clinical immunologic interventions for the treatment of type 1 diabetes. Cold Spring Harbor Perspectives in Medicine. 2012;2(8). doi:https://doi.org/10.1101/cshperspect.a007716.
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2):85–118. https://doi.org/10.1016/S0162-3109(00)00188-0.
Heatwole C, Ciafaloni E. Mycophenolate mofetil for myasthenia gravis: a clear and present controversy. Neuropsychiatr Dis Treat. 2008;4(6):1203–9.
Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care. 2010;33(4):826–32. https://doi.org/10.2337/dc09-1349.
Emer JJ, Claire W. Rituximab: a review of dermatological applications. J Clin Aesthet Dermatol. 2009;2(5):29–37.
Randall KL. Rituximab in autoimmune diseases. Aust Prescr. 2016;39(4):131–4. https://doi.org/10.18773/austprescr.2016.053.
Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, et al. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37(2):453–9. https://doi.org/10.2337/dc13-0626.
Mayer E, Holzl M, Ahmadi S, Dillinger B, Pilat N, Fuchs D, et al. CTLA4-Ig immunosuppressive activity at the level of dendritic cell/T cell crosstalk. Int Immunopharmacol. 2013;15(3):638–45. https://doi.org/10.1016/j.intimp.2013.02.007.
Vital EM, Emery P. Abatacept in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2006;2(4):365–75.
Melvin G, Sandhiya S, Subraja K. Belatacept: a worthy alternative to cyclosporine? J Pharmacol Pharmacother. 2012;3(1):90–2. https://doi.org/10.4103/0976-500x.92499.
Tooley JE, Waldron-Lynch F, Herold KC. New and future immunomodulatory therapy in type 1 diabetes. Trends Mol Med. 2012;18(3):173–81. https://doi.org/10.1016/j.molmed.2012.01.001.
Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9. https://doi.org/10.1016/s0140-6736(11)60886-6.
Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27(1):55–62. https://doi.org/10.1093/intimm/dxu102.
Tack CJ, Kleijwegt FS, Van Riel PL, Roep BO. Development of type 1 diabetes in a patient treated with anti-TNF-alpha therapy for active rheumatoid arthritis. Diabetologia. 2009;52(7):1442–4. https://doi.org/10.1007/s00125-009-1381-0.
Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244–9. https://doi.org/10.2337/dc09-0054.
Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol (Baltimore, Md : 1950). 2011;187(4):2015–22. https://doi.org/10.4049/jimmunol.1100713.
Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692–8. https://doi.org/10.1056/NEJMoa012864.
Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–608. https://doi.org/10.1056/NEJMoa043980.
Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132(2):166–73. https://doi.org/10.1016/j.clim.2009.04.007.
Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes. 2013;62(11):3901–8. https://doi.org/10.2337/db13-0236.
Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–94. https://doi.org/10.1038/sj.leu.2404683.
Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32(4):488–99. https://doi.org/10.1016/j.immuni.2010.04.002.
• Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59(6):1153–61. https://doi.org/10.1007/s00125-016-3917-4. This study discusses the findings observed from a 2-year randomised trial on antithymocyte globulin in new-onset type 1 diabetes.
Coppieters KT, Harrison LC, von Herrath MG. Trials in type 1 diabetes: antigen-specific therapies. Clin Immunol. 2013;149(3):345–55. https://doi.org/10.1016/j.clim.2013.02.002.
Clemente-Casares X, Tsai S, Huang C, Santamaria P. Antigen-specific therapeutic approaches in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(2):a007773. https://doi.org/10.1101/cshperspect.a007773.
Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991;88(22):10252–6.
Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184(6):2167–74.
Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial--type 1. Diabetes Care. 2005;28(5):1068–76.
Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60(4):1237–45. https://doi.org/10.2337/db10-1360.
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Writing committee for the type 1 diabetes TrialNet Oral insulin study group, effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA. 2017;318(19):1891–902.
Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183(4):1561–7.
Ludvigsson J, Cheramy M, Axelsson S, Pihl M, Akerman L, Casas R. GAD-treatment of children and adolescents with recent-onset type 1 diabetes preserves residual insulin secretion after 30 months. Diabetes Metab Res Rev. 2014;30(5):405–14. https://doi.org/10.1002/dmrr.2503.
Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378(9788):319–27. https://doi.org/10.1016/s0140-6736(11)60895-7.
Ludvigsson J, Hjorth M, Cheramy M, Axelsson S, Pihl M, Forsander G, et al. Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial. Diabetologia. 2011;54(3):634–40. https://doi.org/10.1007/s00125-010-1988-1.
Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta. 2013;1833(2):410–6. https://doi.org/10.1016/j.bbamcr.2012.02.019.
• Juwono J, Martinus RD. Does Hsp60 provide a link between mitochondrial stress and inflammation in diabetes mellitus. J Diabetes Res. 2016;2016:8017571. https://doi.org/10.1155/2016/8017571. This review summarises the known relationships between the expression of heat shock protein 60 and type 1 diabetes.
Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990;87(4):1576–80.
Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23(4):292–8. https://doi.org/10.1002/dmrr.712.
Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2007;23(4):286–91. https://doi.org/10.1002/dmrr.711.
Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70(4):326–36. https://doi.org/10.1111/j.1365-3083.2009.02308.x.
Curotto de Lafaille MA, Muriglan S, Sunshine MJ, Lei Y, Kutchukhidze N, Furtado GC, et al. Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes. J Exp Med. 2001;194(9):1349–59.
Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199(12):1679–88. https://doi.org/10.1084/jem.20032121.
Li Z, Li D, Tsun A, Li B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol. 2015;12(5):558–65. https://doi.org/10.1038/cmi.2015.10.
Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol (Baltimore, Md : 1950). 2008;181(7):4516–22.
Cabrera SM, Rigby MR, Mirmira RG. Targeting regulatory T cells in the treatment of type 1 diabetes mellitus. Curr Mol Med. 2012;12(10):1261–72.
Okubo Y, Torrey H, Butterworth J, Zheng H, Faustman DL. Treg activation defect in type 1 diabetes: correction with TNFR2 agonism. Clin Transl Immunol. 2016;5(1):e56. https://doi.org/10.1038/cti.2015.43.
Wu L, Dakic A. Development of dendritic cell system. Cell Mol Immunol. 2004;1(2):112–8.
Shang N, Figini M, Shangguan J, Wang B, Sun C, Pan L, et al. Dendritic cells based immunotherapy. Am J Cancer Res. 2017;7(10):2091–102.
Marin-Gallen S, Clemente-Casares X, Planas R, Pujol-Autonell I, Carrascal J, Carrillo J, et al. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol. 2010;160(2):207–14. https://doi.org/10.1111/j.1365-2249.2009.04082.x.
Creusot RJ, Giannoukakis N, Trucco M, Clare-Salzler MJ, Fathman CG. It's time to bring dendritic cell therapy to type 1 diabetes. Diabetes. 2014;63(1):20–30. https://doi.org/10.2337/db13-0886.
Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34(9):2026–32. https://doi.org/10.2337/dc11-0472.
Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196(6):851–7.
Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8(12):1142–8. https://doi.org/10.1038/sj.embor.7401099.
Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–3. https://doi.org/10.1038/nature04790.
Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–8.
Diaz-de-Durana Y, Lau J, Knee D, Filippi C, Londei M, McNamara P, et al. IL-2 immunotherapy reveals potential for innate beta cell regeneration in the non-obese diabetic mouse model of autoimmune diabetes. PLoS One. 2013;8(10):e78483. https://doi.org/10.1371/journal.pone.0078483.
Luzina IG, Keegan AD, Heller NM, Rook GA, Shea-Donohue T, Atamas SP. Regulation of inflammation by interleukin-4: a review of "alternatives". J Leukoc Biol. 2012;92(4):753–64. https://doi.org/10.1189/jlb.0412214.
Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178(1):87–99.
Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, et al. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol (Baltimore, Md : 1950). 1997;159(10):4686–92.
Cameron MJ, Arreaza GA, Waldhauser L, Gauldie J, Delovitch TL. Immunotherapy of spontaneous type 1 diabetes in nonobese diabetic mice by systemic interleukin-4 treatment employing adenovirus vector-mediated gene transfer. Gene Ther. 2000;7(21):1840–6. https://doi.org/10.1038/sj.gt.3301309.
Elhelu MA. The role of macrophages in immunology. J Natl Med Assoc. 1983;75(3):314–7.
Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep. 2017;7:40144. https://doi.org/10.1038/srep40144.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Rep. 2014;6:13. https://doi.org/10.12703/p6-13.
Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61(11):2881–92. https://doi.org/10.2337/db11-1635.
Culina S, Boitard C, Mallone R. Antigen-based immune therapeutics for type 1 diabetes: magic bullets or ordinary blanks? Clin Dev Immunol. 2011;2011:286248–15. https://doi.org/10.1155/2011/286248.
Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61(9):2340–8. https://doi.org/10.2337/db12-0049.
Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF type 1 diabetes combination therapy assessment group. Clin Exp Immunol. 2010;160(2):176–84. https://doi.org/10.1111/j.1365-2249.2010.04153.x.
Lord S, Greenbaum CJ. Disease modifying therapies in type 1 diabetes: where have we been, and where are we going? Pharmacol Res. 2015;98:3–8. https://doi.org/10.1016/j.phrs.2015.02.002.
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler. 2012;18(12):1721–36. https://doi.org/10.1177/1352458512441381.
Faucard R, Madeira A, Gehin N, Authier FJ, Panaite PA, Lesage C, et al. Human endogenous retrovirus and neuroinflammation in chronic inflammatory demyelinating polyradiculoneuropathy. EBioMedicine. 2016;6:190–8. https://doi.org/10.1016/j.ebiom.2016.03.001.
•• Levet S, Medina J, Joanou J, Demolder A, Queruel N, Reant K et al. An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight. 2017;2(17). doi:https://doi.org/10.1172/jci.insight.94387. The study reports on the potential of human endogenous retroviruses as therapeutic targets in the potential cure of type 1 diabetes.
Curtin F, Bernard C, Levet S, Perron H, Porchet H, Medina J, et al. A new therapeutic approach for type 1 diabetes: rationale for GNbAC1, an anti-HERV-W-Env monoclonal antibody. Diabetes Obes Metab. 2018;20(9):2075–84. https://doi.org/10.1111/dom.13357.
Rewers M, Gottlieb P. Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care. 2009;32(10):1769–82. https://doi.org/10.2337/dc09-0374.
von Herrath M, Peakman M, Roep B. Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol. 2013;172(2):186–202. https://doi.org/10.1111/cei.12085.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Therapies and New Technologies in the Treatment of Diabetes
Rights and permissions
About this article
Cite this article
Xin, G.L.L., Khee, Y.P., Ying, T.Y. et al. Current Status on Immunological Therapies for Type 1 Diabetes Mellitus. Curr Diab Rep 19, 22 (2019). https://doi.org/10.1007/s11892-019-1144-3
Published:
DOI: https://doi.org/10.1007/s11892-019-1144-3