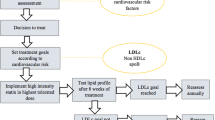
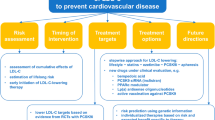
Abstract
Low-density lipoproteins (LDL) play a causal role in the development of atherosclerosis, and reduction of LDL cholesterol with a statin is a cornerstone in prevention of cardiovascular disease. However, it remains an unmet need to reduce the residual risk on maximally tolerated statin alone or in combination with other drugs such as ezetimibe. Among the new LDL-lowering therapies, PCSK9 inhibitors appear the most promising class. Genetic studies suggest that triglyceride-rich lipoproteins are associated with cardiovascular risk and several promising triglyceride-lowering therapies are at various stages of development. At the opposite end, high-density lipoprotein (HDL) cholesterol seems to not be causally associated with cardiovascular risk, and thus far, trials designed to reduce cardiovascular risk by mainly raising HDL cholesterol levels have been disappointing. Nevertheless, new drugs targeting HDL are still in development. This review describes the new drugs reducing LDL, apolipoprotein(a), and triglyceride-rich lipoproteins, and the strategies to modulate HDL metabolism.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Catapano AL, Ference BA. IMPROVE-IT and genetics reaffirm the causal role of LDL in cardiovascular disease. Atherosclerosis. 2015;241:498–501.
Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33:1635–701.
Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events. J Am Coll Cardiol. 2014;64:485–94.
Cannon CP, Blazing MA, Giugliano RP, for the IMPROVE-IT investigators, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy - European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–22.
Seidah NG, Awan Z, Chrétien M, et al. PCSK9. A key modulator of cardiovascular health. Circ Res. 2014;114:1022–36.
Norata GD, Tibolla G, Catapano AL. PCSK9 inhibition for the treatment of hypercholesterolemia: promises and emerging challenges. Vascul Pharmacol. 2014;62:103–11.
Marais AD, Kim JB, Wasserman SM, et al. PCSK9 inhibition in LDL cholesterol reduction: genetics and therapeutic implications of very low plasma lipoprotein levels. Pharmacol Ther. 2015;145:58–66.
Shimada YJ, Cannon CP. PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur Heart J. 2015;36:2415–24.
Bergeron N, Phan BAP, Ding Y, et al. Proprotein convertase subtilisin/kexin type 9 inhibition. A new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132:1648–66. A recent comprehensive review on PCSK9 function and PCSK9 inhibition.
Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–33.
Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Cohen JC, Boerwinkle E, Mosley Jr TH, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Benn M, Nordestgaard BG, Grande P, et al. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42.
Hedrick JA. Targeting PCSK9 for the treatment of hypercholesterolemia. Curr Opin Invest Drugs. 2009;10:938–46.
Turner T, Stein EA. Non-statin treatments for managing LDL cholesterol and their outcomes. Clin Ther. 2015;37:2751–69. Good review on new treatments targeting LDL metabolism.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8.
Fitzgerald K, Simon A, White S, et al. ALN-PCSsc, an RNAi investigational agent that inhibits PCSK9 synthesis with the potential for effective bi-annual dosing. Presented at AHA scientific session 2015.
Galabova G, Brunner S, Winsauer G, et al. Peptide-based anti-PCSK9 vaccines – An approach for long-term LDLc management. PLoS One. 2014;9:e114469.
Farnier M. An evaluation of alirocumab for the treatment of hypercholesterolemia. Expert Rev Cardiovasc Ther. 2015;13:1307–23. Recent review on alirocumab.
Langslet G, Emery M, Wasserman SM. Evolocumab (AMG 145) for primary hypercholesterolemia. Expert Rev Cardiovasc Ther. 2015;13:477–88. Recent review on evolocumab.
Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging, study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21.
Kastelein JJP, Nissen SE, Rader DJ, et al. Safety and efficacy of LY3015014, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9): a randomized, placebo-controlled phase 2 study. Eur Heart J. 2016; Jan 12: online.
Kastelein JJP, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78-week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolemia. Eur Heart J. 2015;36:2996–3003. Studies evaluating efficacy of alirocumab in two large cohorts of heterozygous FH.
Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolemia (RUTHERFORD-2): a randomized, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40.
Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B); a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. Study showing a significant effect of evolocumab for homozygous FH with residual LDL receptor activity.
Robinson JG, Farnier M, Krempf M, et al. For the ODYSSEY LONG TERM Investigators. Efficacy and safety of Alirocumab in reducing lipids and cardiovascular events. New Engl J Med. 2015;372:1489–99. Important phase III trial evaluating long term efficacy and safety of alirocumab, with a post-hoc analysis suggesting a cardiovascular benefit.
Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906–15.
Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–94.
Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia. JAMA. 2014;311:1870–82.
Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–19.
Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–46.
Bays H, Gaudet D, Weiss R, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140–8.
Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61.
Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia – the MENDEL-2 randomized, controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–40.
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8.
Moriarty PM, Thompson PD, Cannon CP, for the ODYSSEY ALTERNATIVE Investigators, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–69.
Sabatine MS, Giugliano RP, Wiviott SD, for the Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. Important phase III trial suggesting a cardiovascular benefit after one year of evolocumab treatment.
Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia. A systematic review and meta-analysis. Ann Intern Med. 2015;163:40–51.
Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37:536–45.
Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER) trial. Am Heart J. 2016;173:94–101.
Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–9.e1.
SPIRE-1: The evaluation of Bococizumab (PF-04950615; RN316) in reducing the occurrence of major cardiovascular events in high risk subjects. www.clinicaltrials.gov, NCT 01975376, accessed on February 2016.
SPIRE-2: The evaluation of Bococizumab (PF-04950615; RN316) in reducing the occurrence of major cardiovascular events in high risk subjects. www.clinicaltrials.gov, NCT 01975389, accessed on February 2016.
EBBINGHAUS: Evaluating PCSK9 Binding antiBody Influence oN cognitive HeAlth in High cardiovascular Risk Subjects. www.clinicaltrials.gov, NCT02207634, accessed on February 2016.
Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field ? J Am Coll Cardiol. 2015;65:2638–51.
Tice JA, Kazi DS, Pearson SD. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for treatment of high cholesterol levels. Effectiveness and value. JAMA Intern Med. 2016;176:107–8.
Agarwala A, Jones P, Nambi V. The role of antisense oligonucleotide therapy in patients with familial hypercholesterolemia: risks, benefits, and management recommendations. Curr Atheroscler Rep. 2015;17:467.
Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36:566–75.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006.
Ahmad Z, Khera A. The role of microsomal triglyceride transfer protein inhibitors in the treatment of patients with familial hypercholesterolemia: risks, benefits, and management. Curr Atheroscler Rep. 2015;17:469.
Cuchel M, Meagher EA, du Toit Theron H, for the phase 3 HoFH Lomitapide Study Investigators, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6.
Stefanutti C, Blom DJ, Averna MR, for the phase 3 HoFH Lomitapide Study Investigators, et al. The lipid-lowering effects of lomitapide are unaffected by adjunctive apheresis in patients with homozygous familial hypercholesterolaemia – A post-hoc analysis of a Phase 3, single-arm, open-label trial. Atherosclerosis. 2015;240:408–14.
Gutierrez MJ, Rosenberg NL, MacDougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–83.
Ballantyne CM, Davidson MH, MacDougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia. Results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62:1154–62.
Thompson P, Ballantyne C, McKenney J, et al. ETC-1002 lowers LDL-C more than ezetimibe in patients with hypercholesterolemia with or without statin intolerance and has a similar safety and tolerability profile. J Am Coll Cardiol. 2015;65:A345.
Bays HE, McKenney JM, Dujovne CA, et al. Effectiveness and tolerance of a new lipid-altering agent, gemcabene, in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol. 2003;92:538–43.
Donovan JM, Mancini M, Sanabria C, et al. Phase 1 study of CAT-2054, an oral modulator of SREBP. J Clin Lipidol. 2015;9:474–5.
Nordestgaard B, Varbo A. Lipids and cardiovascular disease 3. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–35.
Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000.
Miller M, Cannon CP, Murphy SA, for the PROVE-IT-TIMI 22 Investigations, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE-IT TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30.
Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–75.
Varbo A, Benn M, Tybjærg-Hansen A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–36.
Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–50.
Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–39.
Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, et al. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41.
The TG and HDL Working Group off the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides and coronary disease. N Engl J Med. 2014;371:22–31.
Gryn SE, Hegele RA. Novel therapeutics in hypertriglyceridemia. Curr Opin Lipidol. 2015;26:484–91.
Sahebkar A, Chew GT, Watts GF. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog Lipid Res. 2014;56:47–66.
REDUCE-IT: A study of AMR101 to evaluate its ability to reduce cardiovascular events in high risk patients with hypertriglyceridemia and on statin. www.clinicaltrials.gov, NCT 01492361, accessed on February 2016.
STRENGTH: Outcomes study to assess statin residual risk reduction with Epanova in high CV risk patients with hypertriglyceridemia. www.clinicaltrials.gov, NCT 02104817, accessed on February 2016.
Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–6. Proof-of-concept trial demonstrating ApoC3 inhibition would be effective to treat hyperchylomicronemia.
Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–47. Important trial showing positive effects of ASO apoC3 inhibitor.
Gusarova V, Alexa CA, Wang Y, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56:1308–17.
Wang Y, Gusarova V, Banfi S, et al. Inactivation if ANGPTL3 reduces hepatic VLDL- triglyceride secretion. J Lipid Res. 2015;56:1296–307.
Liu ZM, Hu M, Chan P, et al. Early investigational drugs targeting PPAR-alpha for the treatment of metabolic disease. Expert Opin Investig Drugs. 2015;24:611–21.
Ferreira W, Twisk J, Kwikkers K, et al. Immune responses to intramuscular administration of alipogene tiparvocec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25:180–8.
Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80.
Nordestgaard BG, Tybjærg-Hansen A. Genetic determinants of LDL, lipoprotein(a), triglyceride-rich lipoproteins and HDL: concordance and discordance with cardiovascular disease risk. Curr Opin Lipidol. 2011;22:113–22.
Kingwell BA, Chapman MJ, Kontush A, et al. HDL-targeted therapies: progress, failures and future. Nat Rev. 2014;13:445–64.
Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–13.
Gautier T, Masson D, Lagrost L. The potential of cholesteryl ester transfer protein as a therapeutic target. Exper Opin Ther Targets. 2016;20:47–59. Excellent review on the putative role of CETP inhibition.
Kastelein JJP, Besseling J, Shah S, et al. Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomized, double-blind, placebo controlled, phase 3 study. Lancet. 2015;385:2153–61.
Howingh GK, Kastelein JP, van Deventer SJH, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP); a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386:452–60.
REVEAL: Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification. www.clinicaltrials.gov, NCT 01252953, accessed on February 2016.
Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300.
Kootte RS, Smits LP, van der Valk FM, et al. Effect of open-label infusion of an apoA-1-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res. 2015;56:703–12.
Hovingh GK, Smits LP, Stefanutti C, et al. The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: the Modifying Orphan Disease Evaluation (MODE) study. Am Heart J. 2015;169:736–42.e1.
Gille A, Easton R, D’Andrea D, et al. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–14.
Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9.
Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the Phase 2b, randomized, double-blind, multicenter, ASSURE trial. Am J Cardiovasc Drugs. 2016;16:55–65.
Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88:1294–311.
Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53.
Van Capelleveen JC, van der Valk FM, Stroes ESG. Current therapies for lowering lipoprotein(a). J Lipid Res. 2015 Dec 4.
Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–83. First-trial with an ASO blocking apo(a) synthesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Farnier reports having received grants, consulting fees, and/or honoraria, and delivering lectures for Abbott/Mylan, Amgen, AstraZeneca, Eli Lilly, Genzyme, Kowa, Merck and Co, Pfizer, Roche, Sanofi/Regeneron, and Servier.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lipid Abnormalities and Cardiovascular Prevention
Rights and permissions
About this article
Cite this article
Farnier, M. Future Lipid-Altering Therapeutic Options Targeting Residual Cardiovascular Risk. Curr Cardiol Rep 18, 65 (2016). https://doi.org/10.1007/s11886-016-0743-8
Published:
DOI: https://doi.org/10.1007/s11886-016-0743-8