Abstract
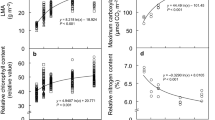
This study of animal–plant interaction focused on the impact of oviposition by an insect on the leaves of Prunus avium (cherries). We examined whether the oviposition by Caliroa cerasi affects leaf mechanical and spectral traits in P. avium. Three cultivars of P. avium were studied. Infested leaves had from 1 to 18 eggs and exhibited higher leaf dry mass per area (LMA) than leaves without eggs. Leaf dry weight and LMA were positively correlated with egg number per leaf. Infested leaves tended to have higher number of trichomes. Leaf thickness and material and structural resistance tended to increase in infested leaves. The reflectance across all wavelengths (500–700 nm) in leaves with larger number of eggs was higher compared to leaves without eggs. Photosynthetic performance was reduced and oxidative activity was increased in leaves with eggs. Extrafloral nectaries increased with increasing the number of eggs per leaf and thus play an important role in defense against herbivores by providing nectar rewards that attract their depredators. These responses to oviposition may be beneficial for the plants in terms of resistance to feeding larvae.







Similar content being viewed by others
References
Agrawal A (1998) Induced responses to herbivory and increased plant performance. Science 279:1201–1202
Amo L, Jansen JJ, Dam NM, Dicke M, Visser ME (2013) Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol Lett 16:1348–1355
Bado S (2007) Plagas del cultivo de cerezo. Revista Fruticultura Profesional 171:14–22
Baldini E, Facini O, Nerozzi F, Rossi F, Rotondi A (1997) Leaf characteristics and optical properties of different woody species. Trees 12:73–81
Beeskow A, Del Valle H, Rostagno C (1987) Los sistemas fisiográficos de la región árida y semiárida de la provincia del Chubut. SECYT, Puerto Madryn
Bittner N, Trauer-Kizilelma U, Hilker M (2017) Early plant defence against insect attack: involvement of reactive oxygen species in plant responses to insect egg deposition. Planta 245:993–1007
Blackmer T, Schepers J, Varvel G (1994) Light reflectance compared with other nitrogen stress measurements in corn leaves. Agron J 86:934–938
Bown A, Hall D, MacGregor K (2002) Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol 129:1430–1434
Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165:373–389. https://doi.org/10.1111/j.1469-8137.2004.01263.x
Bronstein J (1998) he contribution of ant-plant protection studies to our understanding of mutualism. Biotropica 30:150–161
Büchel K, McDowell E, Nelson W, Descour A, Gershenzon J, Hilker M, Soderlund C, Gang DR, Fenning T, Meiners T (2012) An elm EST database for identifying leaf beetle egg induced defense genes. BMC Genom 13:242
Carl K (1972) On the biology, ecology and population dynamic of Caliroa cerasi (L.) (Hym., Tenthredinidae). Z Angew Entomol 71:58–83
Chehab W, Yao C, Henderson Z, Kim S, Braam J (2012) Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol 22:701–706
Cittadini E (2007) Sweet cherries from the end of the world: Options and constraints for fruit production systems in South Patagonia, Argentina. PhD thesis. Wageningen University, The Netherlands
Cittadini E, San Martino L (2007) El cultivo de cerezos en Patagonia Sur Tecnología de manejo, empaque y comercialización. Ediciones INTA 200
Codella S, Raffa K (2002) Desiccation of Pinus foliage induced by conifer sawfly oviposition: effect on egg viability. Ecol Entomol 27:618–621
Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on Bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
Coley P (1983) Herbivory and defensive characteristics of tree species in a lowland tropical rain forest. Ecol Monogr 53:209–233
Dalin P, Björkman C (2003) Adult beetle grazing induces willow trichome defense against subsequent larval feeding. Oecologia 134:112–118
Dalin P, Ågren J, Björkman C, Huttunen P, Kärkkäinen K (2008) Leaf trichome formation and plant resistance to herbivory. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, Dordrecht, pp 89–105
Délano-Frier J, Sánchez-Hernández C, Tiessen A (2012) Friend or foe? Exploring the factors that determine the difference between positive and negative effects on photosynthesis in response to insect herbivory. In: Najafpour M (ed) Artificial photosynthesis. InTech, New York, pp 155–206
Escobar-Bravo R, Klinkhamer PG, Leiss KA (2017) Induction of jasmonic acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol 58:622–634
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607. https://doi.org/10.1371/journal.pone.0043607
Fornoff F, Gross E (2014) Induced defense mechanisms in an aquatic angiosperm to insect herbivory. Oecologia 175:173–185. https://doi.org/10.1007/s00442-013-2880-8
Gish M, Mescher MC, De Moraes CM (2016) Mechanical defenses of plant extrafloral nectaries against herbivory. Commun Integr Biol 9:e1178431
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178
Heil M (2009) Damaged-self recognition in plant herbivore defence. Trends Plant Sci 14:356–363. https://doi.org/10.1016/j.tplants.2009.04.002
Heong KL, Cheng J, Escalada MM (2015) Rice planthoppers. Ecology, Management, Socio Economics and Policy. Advanced Topics in Science and Technology in China. Zhejiang University Press, Hangzhou and Springer, Berlin Heidelberg
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515
Hilker M, Fatouros NE (2016) Resisting the onset of herbivore attack: plants perceive and respond to insect eggs. Curr Opin Plant Biol 32:9–16
Hilker M, Meiners T (2006) Early herbivore alert: insect eggs induce plant defense. J Chem Ecol 32:1379–1397
Hilker M, Bläske V, Kobs C, Dippel C (2002) Kairomonal effects of sawfly sex pheromones on egg parasitoids. J Chem Ecol 26:2591–2601
Karban R, Baldwin I (1997) Induced responses to herbivory. The University of Chicago Press, Chicago, IL, p. 330
Koski TM, Lindstedt C, Klemola T, Troscianko J, Mäntylä E, Tyystjärvi E, Stevens M, Helander M, Laaksonen T (2017) Insect herbivory may cause changes in the visual properties of leaves and affect the camouflage of herbivores to avian predators. Behav Ecol Sociobiol 71:97
Little D, Gouhier-Darimont C, Bruessow F, Reymond P (2007) Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol 143:784–800. https://doi.org/10.1104/pp.106.090837
Liu Y, Schieving F, Stuefer J, Anten N (2007) The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Ann Bot 99:121–130. https://doi.org/10.1093/aob/mcl230
Liu Z, Cai Y, Fang Y, Jing J, Li K (2010) Induced response in Schima superba: Effects of early-season herbivory on leaf traits and subsequent insect attack. Afr J Biotechnol 9:8731–8738
Lucas-Barbosa D, van Loon J, Gols R, van Beek T, Dicke M (2012) Reproductive escape: annual plant responds to butterfly eggs by accelerating seed production. Funct Ecol 27:245–254
Mäntylä E, Klemola T, Haukioja E (2004) Attraction of willow warblers to sawfly-damaged mountain birches: novel function of inducible plant defences? Ecol Lett 7:915–918
Mäntylä E, Klemola T, Sirkiä P, Laaksonen T (2007) Low light reflectance may explain the attraction of birds to defoliated trees. Behav Ecol 19:325–330
Mareggiani G, Bartoloni N, Gorosito N, Laffaye C (2012) Flight detection of Caliroa cerasi L. (Hymenoptera: Tenthredinidae) adults in the Andean Region of Parallel 42, Argentina. Bol San Veg Plagas 38:233–238
Markovic D, Glinwood R, Olsson U, Ninkovic V (2014) Plant response to touch affects the behaviour of aphids and ladybirds. Arth-Plant Int 8:171–181
Miller G, Miller E (1948) Determination of nitrogen in biological materials. Anal Chem 20:481–488
Mitchell C, Brennan RM, Graham J, Karley AJ (2016) Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Front Plant Sci 7:1132
Oliveira P (1997) The ecological function of extrafloral nectaries: herbivore deterrence by visiting ants and reproductive output in Caryocar brasiliense (Caryocaraceae). Funct Ecol 11:323–330
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Onoda Y, Westoby M, Adler P, Choong A, Clissold F, Cornelissen J et al (2011) Global patterns of leaf mechanical properties. Ecol Lett 14:301–312. https://doi.org/10.1111/j.1461-0248.2010.01582.x
Pashalidou FG (2015) Getting prepared for future attack: induction of plant defences by herbivore egg deposition and consequences for the insect community. Wageningen University
Pashalidou F, Lucas-Barbosa D, van Loon J, Dicke M, Fatouros N (2013) Phenotypic plasticity of plant response to herbivore eggs: effects on resistance to caterpillars and plant development. Ecology 94:702–713
Peñaflor M, Erb M, Miranda L, Werneburg A, Bento J (2011) Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Soc 37:1304–1313
Peñuelas J, Munné-Bosch S, Llusià J, Filella I (2004) Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia Optical signals of oxidative stress? New Phytol 162:115–124
Peschiutta M (2015) El impacto de la herbivoría por Caliroa cerasi (Hymenoptera: Tenthredinidae) sobre las relaciones hídricas, intercambio gaseoso, crecimiento y productividad del cerezo (Prunus avium L.). Universidad de Buenos Aires
Peschiutta ML, Bucci SJ, Scholz FG, Goldstein G (2016) Compensatory responses in plant-herbivore interactions: impacts of insects on leaf water relations. Acta Oecol 73:71–79
Peschiutta ML, Scholz FG, Goldstein G, Bucci SJ (2018) Herbivory alters plant carbon assimilation, patterns of biomass allocation and nitrogen use efficiency. Acta Oecol 86:9–16
Petzold-Maxwell J, Wong S, Arellano C, Gould F (2011) Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa. Ecol Entomol 36:700–708
Poorter H, Niinemets Ü, Poorter L, Wright I, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Pulice C, Packer A (2008) Simulated herbivory induces extrafloral nectary production in Prunus avium. Funct Ecol 22:801–807
Raffa K, Lintereur G (1988) New host records and developmental notes on the pear slug Caliroa cerasi (Hymenoptera: Tenthredinidae), feeding on Cotoneaster and Chaenomeles species Great Lakes. Entomol 21:75–79
Sack L, Cowan P, Jaikumar N, Holbrook N (2003) The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant Cell Environ 26:1343–1356
Schlesinger W, Hasey M (1981) Decomposition of chaparral shrub foliage: losses of organic and inorganic constituents from deciduous and evergreen leaves. Ecology 62:762–774
Schröder R, Forstreuter M, Hilker M (2005) A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol 138:470–477
Shapiro AM, DeVay JE (1987) Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 71:631–632. https://doi.org/10.1007/BF00379310
Shaw P, Wallis D, Alspach P, Bus V, Brewer L (2004) Pear sawfly (Caliroa cerasi) (Hymenoptera: Tenthredinidae) host preference and larval development on six Pyrus genotypes. New Zeal J Crop Hort 32:257–262
Slaton M, Hunt E, Smith W (2001) Estimating near-infrared leaf reflectance from leaf structural characteristics. Am J Bot 88:278–284
Telewski F (2006) A unified hypothesis of mechanoperception in plants. Am J Bot 93:1466–1476. https://doi.org/10.3732/ajb.93.10.1466
Velikova V, Salerno G, Frati F, Peri E, Conti E, Colazza S, Loretto F (2010) Influence of feeding and oviposition by phytophagous pentatomids on photosynthesis of herbaceous plants. J Chem Ecol 36:629–641. https://doi.org/10.1007/s10886-010-9801-7
Zangerl A, Hamilton J, Miller T, Crofts A, Oxborough K, Berenbaum M, de Lucia E (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci USA 99:1088–1091
Acknowledgements
We thank Eng. Claudia Mundet and field staff from Bahía Solano S.A. Ranch for logistic support and for permission to access and use sweet cherry plantation for this study. This study was partially supported by CyT Chubut, Argentina, CONICET (PIP Grant), and ANCyT-FONCyT (PICT Grants). This work complies with Argentinean Law.
Author information
Authors and Affiliations
Contributions
MLP, FGS, and GG conceived and designed the experiments. MLP performed the experiments. MLP, FGS, and SJB analyzed the data. MLP, FGS, SJB, and GG wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Handling Editor: Stanislav Gorb.
Rights and permissions
About this article
Cite this article
Peschiutta, M.L., Scholz, F.G., Goldstein, G. et al. Oviposition by herbivorous insects induces changes in optical and mechanical properties of Prunus avium leaves. Arthropod-Plant Interactions 12, 613–622 (2018). https://doi.org/10.1007/s11829-018-9609-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-018-9609-x