Abstract
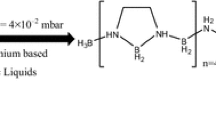
The current work presents a mechanistic insight of hydrogen production from ammonia borane (AB) facilitated by the phosphonium based ionic liquid (IL), trihexyl(tetradecyl)phosphonium bis (2,4,4-trimethylpentyl) phosphinate ([TDTHP][Phosph]). Prior to experiments, the IL was screened from a pool of 11 phosphonium ILs with the infinite dilution activity coefficients (IDAC) values as predicted by conductor like screening model segment activity coefficient (COSMO-SAC) theory. Thereafter, a dehydrogenation experiment of AB/[TDTHP][Phosph] was carried out at 105 °C and 4×10-2 mbar of gauge pressure, which yielded 2.07 equivalent hydrogen production. At higher temperature, the 11B NMR characterization shows the suppression of induction period at 105 °C and appearance of borohydride anion after 1 min of dehydrogenation. Further, time-resolved characterization of AB/[TDTHP][Phosph] at 105 °C confirmed the appearance of polymeric aminoborane after 10min with a subsequent formation of polyborazylene. HR-MS characterization coupled with 1H resonance spectrum confirmed structural integrity of IL. The dual characterization of NMR and HR-MS led us to propose a dehydrogenation mechanism of AB/[TDTHP][Phosph] system.
Similar content being viewed by others
References
T. Richardson, S. de Gala, R. H. Crabtree and P. E. M. Siegbahn, J. Am. Chem. Soc., 117, 12875 (1995).
J. Li, F. Zhao and F. Jing, J. Chem. Phys., 116, 25 (2002).
A. Al-Kukhun, H. T. Hwang and A. Varma, Ind. Eng. Chem. Res., 50, 8824 (2011).
G. Cinti, D. Frattini, E. Jannelli, U. Desideri and G. Bidini, Appl. Energy, 192, 466 (2017).
F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2613 (2007).
A. Rossin and M. Peruzzini, Chem. Rev., 116, 8848 (2016).
A. Gutowska, L. Li, Y. Shin, C. M. Wang, X. S. Li, J. C. Linehan, R. S. Smith, B. D. Kay, B. Schmid, W. Shaw, M. Gutowski and T. Autrey, Angew. Chem., Int. Ed., 44, 3578 (2005).
H.-L. Jiang and Q. Xu, Catal. Today, 170, 56 (2011).
D. W. Himmelberger, L. R. Alden, M. E. Bluhm and L. G. Sneddon, Inorg. Chem., 48, 9883 (2009).
M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 108, 2015 (2008).
T. Welton, Chem. Rev., 99, 2071 (1999).
R. M. Vrikkis, K. J. Fraser, K. Fujita, D. R. MacFarlane and G. D. Elliott, J. Biomech. Eng., 131, 074514 (2009).
C. V. Manohar, D. Rabari, A. A. P. Kumar, T. Banerjee and K. Mohanty, Fluid Phase Equilib., 360, 392 (2013).
M. E. Bluhm, M. G. Bradley, R. Butterick, U. Kusari and L. G. Sneddon, J. Am. Chem. Soc., 128, 7748 (2006).
T. Nakagawa, A. K. Burrell, R. E. Del Sesto, M. T. Janicke, A. L. Nekimken, G. M. Purdy, B. Paik, R.-Q. Zhong, T. A. Semelsberger and B. L. Davis, RSC Adv., 4, 21681 (2014).
R. K. Ahluwalia, J. K. Peng, and T. Q. Hua, Int. J. Hydrogen Energy, 36, 15689 (2011).
S. Mahato, B. Banerjee, G. Pugazhenthi and T. Banerjee, Int. J. Hydrogen Energy, 40, 10390 (2015).
M. J. Valero-Pedraza, A. Martín-Cortés, A. Navarrete, M. D. Bermejo and Á. Martín, Energy, 91, 742 (2015).
S. Gatto, O. Palumbo, F. Trequattrini and A. Paolone, J. Therm. Anal. Calorim., 129, 663 (2017).
N. Sahiner and D. Alpaslan, J. Appl. Polym. Sci., 131, 40183 (2014).
W. R. H. Wright, E. R. Berkeley, L. R. Alden, R. T. Baker and L. G. Sneddon, Chem. Commun., 47, 3177 (2011).
S. S. Mal, F. H. Stephens and R. T. Baker, Chem. Commun., 47, 2922 (2011).
B. D. Rekken, A. E. Carre-Burritt, B. L. Scott and B. L. Davis, J. Mater. Chem. A, 2, 16507 (2014).
R. K. Blundell and P. Licence, Phys. Chem. Chem. Phys., 16, 15278 (2014).
F. Atefi, M. T. Garcia, R. D. Singer and P. J. Scammells, Green Chem., 11, 1595 (2009).
R. E. Del Sesto, C. Corley, A. Robertson and J. S. Wilkes, J. Organomet. Chem., 690, 2536 (2005).
E. Frackowiak, G. Lota and J. Pernak, J., Appl. Phys. Lett., 86, 164104 (2005).
K. Tsunashima, and M. Sugiya, Electrochem. Commun., 9, 2353 (2007).
C. J. Bradaric, A. Downard, C. Kennedy, A. J. Robertson and Y. Zhou, Green Chem., 5, 143 (2003).
C. Zhang, B. Xin, Z. Xi, B. Zhang, Z. Li, H. Zhang, Z. Li and J. Hao, ACS Sustainable Chem. Eng., 6, 1468 (2018).
Y. Shia and B. Zhang, Chem. Soc. Rev., 45, 1529 (2016).
J. F. Callejas, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Chem. Mater., 28, 6017 (2016).
R. Dennington, T. Keith and J. Millam, GaussView (Version 5), Semichem Inc., Shawnee Mission, KS (2009).
M. J. Frisch, et al., Gaussian 09 (Revision D.01), Gaussian, Inc., Wallingford, CT (2013).
A. D. Becke, J. Chem. Phys., 98, 5648 (1993).
C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 37, 785 (1988).
J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 33, 8822 (1986).
C. Sosa, J. Andzelm, B. C. Elkin, E. Wimmer, K. D. Dobbs and D. A. Dixon, J. Phys. Chem., 96, 6630 (1992).
A. Schäfer, H. Horn and R. Ahlrichs, Chem. Phys., 97, 2571 (1992).
A. Bharti, D. Kundu, D Rabari and T. Banerjee, Phase equilibria in ionic liquid facilitated liquid-liquid extractions, CRC Press, New York (2017).
D. Kundu, B. Banerjee, G. Pugazhenthi and T. Banerjee, Int. J. Hydrogen Energy, 42, 2756 (2017).
D. Kundu, S. Chakma, G. Pugazhenthi and T. Banerjee, ACS Omega, 3, 2273 (2018).
A. C. Stowe, W. J. Shaw, J. C. Linehan, B. Schmid and T. Autrey, Phys. Chem. Chem. Phys., 9, 1831 (2007).
N. C. Smythe and J. C. Gordon, Eur. J. Inorg. Chem., 2010, 509 (2010).
S. Sahler, S. Sturm, M. T. Kessler and M. H. G. Prechtl, Chem. Eur. J., 20, 8934 (2014).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kundu, D., Chakma, S., Pugazhenthi, G. et al. Reactive insights into the hydrogen production from ammonia borane facilitated by phosphonium based ionic liquid. Korean J. Chem. Eng. 36, 456–467 (2019). https://doi.org/10.1007/s11814-018-0196-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-018-0196-4