Abstract
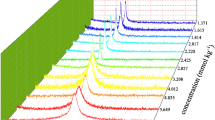
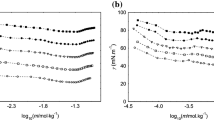
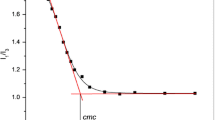
Electrical conductivities of sodium perfluorooctanoate (SPFO) in aqueous solutions were measured at different temperatures (range 294–328 K). Critical micelle concentrations (CMC) and the degree of ionization (α) of the micelles were derived from such data. The results revealed that temperature dependence of CMC is U-shaped with a minimum at 316 K. Gibbs free energies, enthalpies, and entropies of micelle formation as a function of temperature were estimated from the CMC and α values using the charged pseudo-phase separation model. To correlate the enthalpic and entropic contributions, the compensation phenomenon was studied, with a compensation temperature of 309 K and an intercept of −27.7 kJ·mol−1. Apparent molar volumes and adiabatic compressibilities of SPFO were determined from density and ultrasound velocity measurements in the same temperature range as conductivities. Positive deviation from the Debye-Hückel limiting law of the apparent molar volume in the range of temperatures studied evidenced hydrogen bonding-type interactions between monomers and the existence of dimers in the premicellar region. With micellization, the apparent molar volumes decrease with rising temperature, indicating that the structure of micelles is looser than that of monomers. The isentropic apparent molar adiabatic compressibilities following micellization were positive, indicating the predominant role of the decrease in hydrophobic hydration in the association process.
Similar content being viewed by others
Abbreviations
- a:
-
degree of ionization
- CMC:
-
critical micelle concentration
- SPFO:
-
sodium perfluorooctanoate
References
Krafft, M.P., Fluorocarbons and Fluorinated Amphiphiles in Drug Delivery and Biomedical Research, Adv. Drug Deliv. Rev. 47:209 (2001).
Bondi, A., Van der Waals Volumes and Radii, J. Phys. Chem. 68:441 (1964).
Eicke, H.F., Modern Trends of Colloid Science in Chemistry and Biology, Birkhauser Verlag, Basel, 1985, p. 148.
Rosen, M.J., Surfactants and Interfacial Phenomena, Wiley, New York, 1978.
Kissa, E., Fluorinated Surfactants, Synthesis, Properties, Applications, Marcel Dekker, New York, 1994, Surfactant Science Series, Vol. 50.
Simmons J.H., Fluorine Chemistry, Academic Press, New York, 1964, p. 133.
Riess, J.G., Highly Fluorinated Systems for Oxygen Transport, Diagnosis and Drug Delivery, Colloids Surf. A 84:33 (1994).
Krafft, M.P., and J.G. Riess, Highly Fluorinated and Colloidal Systems and Their Applications in the Biomedical Field. A Contribution, Biochimie 80:489 (1998).
Riess, J.G., Fascinated by Fluorine, Elsevier, Amsterdam, 2000.
Riess, J.G., and M.P. Krafft, Fluorocarbons and Fluorosurfactants for in vivo Oxygen Transport (blood substitutes), Imaging, and Drug Delivery, Mat. Res. Soc. Bull. 24:42 (1999).
Mukerjee, P., and T. Handa, Adsorption of Fluorocarbon and Hydrocarbon Surfactants to Air—Water, Hexane-Water and Perfluorohexane-Water Interfaces. Relative Affinities of Fluorocarbon-Hydrocarbon Nonideality Effects, J. Phys. Chem. 85:2298 (1981).
Mukerjee, P. Fluorocarbon-Hydrocarbon Interactions in Micelles and Other Assemblies, at Interfaces, and in Solutions, Colloids Surf. A 84:1 (1994).
Schneider, J., C. Erdelen, H. Ringsdorf, and J.F. Rabolt, Structural Studies of Polymers with Hydrophilic Spacer Groups. 2. Infrared Spectroscopy of Langmuir-Blodgett Multilayers of Polymers with Fluorocarbon Side Chains at Ambient and Elevated Temperatures, Macromolecules 22:3475 (1989).
Fung, B.M., D.L. Mamrosh, E.A. O'Rear, C.B. Fresh, and J. Afzal, Unusual Micellar Properties of a New Class of Fluorinated Nonionic Surfactants, J. Phys. Chem. 92:4404 (1988).
Guo, W., T.A. Brown, and B.M. Fung, Micelles and Aggregates of Fluorinated Surfactants, J. Phys. Chem. 95:1829 (1991).
Shinoda, K., M. Hato, and T. Hayashi, The Physicochemical Properties of Aqueous Solutions of Fluorinated Surfactants, J. Phys. Chem. 76:909 (1972).
Mukerjee, P., K. Korematsu, M. Okawauchi, and G. Sugihara, Effect of Temperature on Electrical Conductivity and the Thermodynamics of Micelle Formation, J. Phys. Chem 89:5308 (1985).
Tamaki, K., Y. Ohara, and S. Watanabe, Solution Properties of Sodium Perfluoroalkanoates. Heats of Solution, Viscosity B Coefficients and Surface Tensions, Bull. Chem. Soc. Jpn. 62:2497 (1989).
Kato, S., S. Harada, H. Nakashima, and H. Nomura, Ultrasonic Relaxation and Volumetric Studies of Micelle-Monomer Exchange Process in Aqueous Solutions of Sodium and Cesium Perfluorooctanoates, J. Colloid Interface Sci. 150:305 (1992).
De Lisi, R., A. Inglese, S. Milioto, and A. Pellerito, Demixing of Mixed Micelles. Thermodynamics of Sodium Perfluorooctanoate-Sodium Dodecanoate in Water, Langmuir 13:192 (1997).
De Lisi, R., S. Milioto, A. De Giacomo, and A. Inglese, Thermodynamic Properties of Sodium n-Perfluoroalkanoates in Water and in Water+Cyclodextrins Mixtures, Langmuir 15:5014 (1999).
Fukada, K., Y. Kobayashi, Y. Ota, M. Fujii, T. Kato, and T. Seimiya, Effect of Pressure and Temperature on Adiabatic Compressibility of Aqueous Solutions of Amphiphiles with a Perfluorocarbon Chain, Thermochim. Acta 352–353:189 (2000).
Oelschlaeger, C., G. Waton, E. Buhler, S. J. Candau, and M. E. Cates, Rheological and Light Scattering Studies of Cationic Fluorocarbon Surfactant Solutions at Low Ionic Strength, Langmuir 18:3076 (2002).
Kiselev, V.D., E.A. Kashaeva, M.S. Shihaab, and A.I. Konovalov, Apparent Molar Volumes and Enthalpies of Solution of Tetracyanoethylene in Some Solvent and of Butan-1-ol in n-Octane at Different Concentrations, Mendeleev Commun. 10:43 (2000).
Amararene, A., M. Gindre, J.-H. Le Huerou, W. Urbach, D. Valdez, and M. Walks, Adiabatic Compressibility of AOT [sodium bis (2-ethylhexyl) sulfosuccinate] Reverse Micelles: Analysis of A Simple Model Based on Micellar Size and Volumetric Measurements, Phys. Rev. E 61:682 (2000).
Muzzalupo, R., G. Ranieri, and C. LaMesa, Solution Properties of Alkali Metal Perfluoroalkanoates, Colloids Surf. A 104:327 (1995).
Tamaki, K., S. Watanabe, and Y. Daikyoji, Partial Molar Volumes of Sodium Perfluoroalkanoates and Lithium Perfluoro-1-alkanesulfonates in Aqueous Solutions, Bull. Chem. Soc. Jpn. 63:3861 (1990).
Perron, G., and J.E. Desnoyers, Volumes and Heat Capacities of Sodium Perfluoroalkanoates in Water, J. Chem. Eng. Data 42:172 (1997).
Kharakoz, D.P., Volumetric Properties of Proteins and Their Analogues in Diluted Water Solutions. 1. Partial Volumes of Amino Acids at 15–55 Degrees C, Biophys. Chem. 34:115 (1989).
Kharakoz, D.P., Volumetric Properties of Proteins and Their Analogues in Diluted Water Solutions. 2. Partial Adiabatic Compressibilities of Amino Acids at 15–70°C, J. Phys. Chem. 95:5634 (1991).
Shedlovsky, T., An Equation for Electrolytic Conductance, J. Am. Chem. Soc. 54:1405 (1932).
Chambers, J.F., J.M. Stokes, and R.H. Stokes, Conductances of Sodium and Potassium Chloride Concentrated Aqueous Solutions at 25°, J. Phys. Chem. 60:985 (1956).
Smit, B., K. Essenlink, P.A.J. Hilbers, N.M. van Os, L.A.M. Rupert, and I. Szleifer, Computer Simulations of Surfactants Self-Assembly, Langmuir 9:9 (1993).
Sarmiento, F., J.M. del Rio, G. Prieto, D. Attwood, M.N. Jones, and V. Mosquera, Thermodynamics of Micelle Formation of Chlorhexidine Digluconate, J. Phys. Chem. 99:17628 (1995).
Hoffmann, H., and W. Ulbright, Kinetic and Thermodynamic Measurements of Aggregation of Perfluorinated Surfactants, Z. Phys. Chem. 106:167 (1977).
Zana, R., Ionization of Cationic Micelles: Effect of the Detergent Structure, J. Colloid Interface Sci. 78:330 (1980).
Shinoda, K., and E. Hutchinson, Pseudo-Phase Separation Model for Thermodynamic Calculations on Micellar Solutions, J. Phys. Chem. 66:577 (1962).
Lumry, R., and S. Rajender, Enthalpy-Entropy Compensation Phenomena in Water Solutions of Proteins and Small Molecules: An Ubiquitous Property of Water, Biopolymers 9:1125 (1970).
Chen, L.-J., S.-H. Lin, and C.-C. Huang, Effect of Hydrophobic Chain Length of Surfactants on Enthalpy-Entropy Compensation of Micellization, J. Phys. Chem. B 102:4350 (1998).
Sugihara, G., and M. Hisatomi, Enthalpy-Entropy Compensation Phenomenon Observed for Different Surfactants, J. Colloid Interface Sci. 219:31 (1999).
Desnoyers, J.E., R. de Lis, C. Ostiguy, and G. Perron, Solution Chemistry of Surfactants, Plenum Press, New York, 1979.
Redlich, O., and D.M. Meyer, The Molal Volumes of Electrolytes, Chem. Rev. 64:221 (1964).
Leduc, P.A., J.L. Fortier, and J.E. Desnoyers, Apparent Molal Volumes, Heat Capacities, and Excess Enthalpies of N-Alkylamine Hydrobromides in Water as a Function of Temperature J. Phys. Chem. 78:1217 (1974).
Brun, T.S., H. Høiland, and E. Vikingstad, Partial Molal Volumes and Isentropic Partial Molal Compressibilities of Surface-Active Agents in Aqueous Solution, J. Colloid Interface Sci. 63:89 (1978).
Musbally, G.M., G. Perron, and J.E. Desnoyers, Apparent Molal Volumes and Heat Capacities of Ionic Surfactants in Water at 25°C, J. Colloid Interface Sci. 48:494 (1974).
Zielinski, R., S. Ikeda, H. Nomura, and S. Kato, Temperature Dependence of Adiabatic Compressibility of Aqueous Solutions of Alkyltrimethylammonium Bromides, J. Chem. Soc., Faraday Trans. I 84:151 (1988).
Harned, H.S., and B.B. Owen, Physical Chemistry of Electrolyte Solutions, Chapman and Hall, London 1957.
Franks, F., M.J. Quikenden, J.R. Ravenhill, and H.T. Smith, Volumetric Behavior of Dilute Aqueous Solutions of Sodium Alkyl Sulfates, J. Phys. Chem. 72:2668 (1968).
Høiland, H., and E. Vikingstad, Partial Molal Volumes and Volumes of Ionization of Hydroxycarboxylic Acid in Aqueous Solution at 25, 30, and 35°C, J. Chem. Soc. Faraday Trans I 71: 2007 (1975).
Conway, B.E., and R.E. Verall, Ion-Solvent Size Ratio as a Factor in the Thermodynamics of Electrolytes, J. Phys. Chem. 70:3952 (1966).
Mosquera, V., J.M. del Rio, D. Attwood, M. García, M.N. Jones, G. Prieto, and F. Sarmiento, A Study of the Aggregation Behavior of Hexyltrimethylammonium Bromide in Aqueous Solution, J. Colloid Interface Sci. 206:66 (1998).
Suarez, M.J., J.L. López-Fontán, F. Sarmiento, and V. Mosquera, Thermodynamic Study of the Aggregation Behavior of Sodium n-Hexyl Sulfate in Aqueous Solution, Langmuir 15:5265 (1999).
Zana, R. (ed.), Surfactant Solutions: New Methods of Investigation, Marcel Dekker, New York, Surfactant Science Series, Vol. 22, 1987.
Bradley, D.J., and K.S. Pitzer, Thermodynamics of Electrolytes. 12. Dielectric Properties of Water and Debye-Hückel Parameters to 350°C and 1 kbar, J. Phys. Chem. 83:1599 (1979).
Garney, R., R.J. Boe, R. Mahoney, and T.A. Litovitz, Determination of Electrolyte Apparent Molal Compressibilities at Infinite Dilution Using a High-Precision Ultrasonic Velocimeter, J. Chem. Phys. 50:5222 (1969).
Cabani, S., G. Conti, and E. Matteoli, Adiabatic and Isothermal Molal Compressibilities of Organic Compounds in Water. I. Cyclic and Open-Chain Secondary Alcohols and Ethers, J. Solution Chem. 8:11 (1979).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
González-Pérez, A., Ruso, J.M., Prieto, G. et al. The self-aggregation of sodium perfluorooctanoate in aqueous solution at different temperatures. J Surfact Deterg 7, 387–395 (2004). https://doi.org/10.1007/s11743-004-0323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-004-0323-9