Abstract
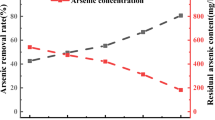
The effect of pressure on the hydrolysis rates and the degradation kinetics of environmentally persistent omethoate pesticide was studied. The results showed that the values of k obs increased and the values of activation volume (AV) decreased with increasing pressure. Among pH conditions (3.58, 6.01 and 8.5), pH 8. 5 was found to be the unstable condition and its half-life was reduced from 263 min at 1 atm to 19.37 min at 10 atm. These results describe that high-pressure hydrolysis is a useful technique for the conversion of toxic organic phosphorus into inorganic phosphorus.
Similar content being viewed by others
References
Ellsworth H E. Toxic Organic Chemicals: Destruction and Wastewater Treatment [M]. Noyes Data Corporation, USA, 1992: 578–583.
Lartiges S B, Garrigues P P. Degradation kinetics of organophosphorous and organonitrogen pesticides in different waters under vaious environmental conditions[J]. Environ. Sci. Technol., 1995, 29:1246–1254.
Andrea D, Simo 0. Investigation of the heterogeneously catalyzed hydrolysis of organophosphorous pesticides[J]. J. Agric. Food Chem., 1998, 46: 325–334.
Siskin M, Katritzky A R. Reactivity of organic compounds in hot water geochemical and technological implicatiuons[J]. Science, 1991, 254: 231–237.
Vacek L. Preperation of 1, 2 diaminobenezene by high pressure acid hydrolysis of benzemidazolone[P]. United States Patent, 4,319,046, 1982.
Franson MAM, Greenberg A E, Trussell R R, et al., Eds. Standard Methods for the Examination of. Water and Wastewater[M]. 16th Ed, American Public Health Association, Washington DC, 1985: 442–450.
Barbra K, Arnett E M, Micheal S. Classical organic reactions in pure superheated water[J]. J. Org. Chem., 1994, 59: 3098–3101.
Feng H, Pehkonen S 0, Elwood B. Pathways for hydrolysis of phorate: Product studies by 31P NMR and GC-MS [J]. J. Org. Chem., 2000, 48:3013–3017.
Silvia L, Damia B. Rapid degration of fenitrothion in estuarine waters [J]. Environ. Sci. Technol., 1994, 28: 1159–116.
Shigeru K, Ichizo K, Kazuya T, et al. Effect of Pressure on subtilisin catalysis. Hydrolysis and peptide synethesis[J]. Bull. Chem. Soc. Jpn., 1996, 69: 3375–3380.
Bradley R S. High Pressure Physics and Chemistry[M]. Academic Press, London, 1963:1–152.
Gibson R E, Leofeler O H. Pressure-volume-temperature relations in solutions. DI. Some thermodynamic properties of mixtures of analine and nitrobenzene [J]. J. Boil. Chem., 1939: 2877–2887.
Lowwitz D A, Spencer J W, Webb W, et al. Temperature-pressure-structure effects on the viscosity of several higher hydrocarbons[J]. Journal of Chemical Physics, 1959, 30(1): 73–83.
Neuman R C, Jr., Kauzmann W, Zipp A. Pressure dependence of weak acid ionization in aqueous buffers [J]. J. Phys. Chem., 1973, 77(22):2687–2690.
Neuman R C, Jr., Pankratz R P. High pressure studies. XV. Polar effects in aliphatic perester decomposition[J]. J. Amer. Chem. Soc., 1973, 95(25): 8372–8374.
Cutler W G, McMickle R H, Webb W, el al. Study of the copressiuons of several high molecular weight hydrocarbons[J]. J. Chem. Phy., 1959, 29(4): 727–740.
Franck E U. Water and aqueous solutions at high pressure and temperature[J]. J. High Pressure and Temperature, 1987, 1:13–27.
Sidney B, Charles V J. High pressure thermal hydrolysis process to decompose triazenes in acid waste streams [P]. United States Patent, 4,013,757, 1977.
Micheal S, Glen B, Stephen N V. Aqueous organic chemistry. 3. aquathermolysis reactivity of ethers and esters[J]. Energy and Fuels, 1990, 4: 488–492.
Shigeru K, Ichizo K, Kazuya T, et al. Effect of pressure on subtilisin catalysis. Hydrolysis and peptide synthesis[J]. Bull. Chem. Soc. Jpn., 1996, 69: 3375–3380.
Feng H, Simo P. Hydrolysis of phorate using simulated environmental conditions rate, mechanism, and product analysis[J]. J. Agric. Food. Chem., 1996, 46: 1192–1199.
Author information
Authors and Affiliations
About this article
Cite this article
Farooq, R., Lin, Fk., Wang, Y. et al. Pressure hydrolysis for degradation of omethoate pesticide in water. J. of Shanghai Univ. 8, 221–226 (2004). https://doi.org/10.1007/s11741-004-0044-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11741-004-0044-0