Abstract
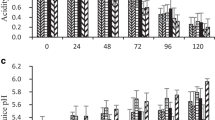
Changes in chemical composition and hydrolytic enzyme activities in guava fruits cv. Lucknow-49 have been reported at four different stages of maturity, viz., mature green (MG), color turning (CT), ripe (R) and over ripe (OR). Chlorophyll content decreased, while carotenoid content increased with advancement of ripening. Starch content decreased with concomitant increase in alcohol soluble sugars. The cell wall constituents viz., cellulose, hemicellulose, and lignin decreased up to R stage, while the pectin content decreased throughout up to OR stage. Among the cell wall hydrolyzing enzymes, polygalacturonase (PG) and cellulase exhibited progressive increase in activity throughout ripening, while pectin methyl esterase (PME) activity increased up to CT stage and then decreased up to OR stage. The maximum increase in the activities of cell wall hydrolysing enzymes was observed between MG and CT stages. The activities of starch hydrolyzing enzymes, α-amylase and β-amylase decreased significantly with advancement of ripening. These changes in the activities of hydrolyzing enzymes could be considered good indicators of ripening in guava.
Similar content being viewed by others
Abbreviations
- MG :
-
mature green
- CT :
-
colour turning
- R :
-
ripe
- OR :
-
over ripe
- PG :
-
polygalacturonase
- PME :
-
pectin methyl esterase
- Chl :
-
chlorophyll
References
Abeles F.B., L.J. Dunn, P. Morgan, A. Callahan, R.E. Dinterman, R.E. Schmidt and J. Schmidt, 1988. Induction of 33 KD and 60KD peroxidase during ethylene induced senescence of cucumber cotyledons. Plant Physiol. 87: 609–615.
Ahmed A.E. and J.M. Labavitch, 1977. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1: 361–365.
Ahmed A.E.R. and J.M. Labavitch, 1980. Cell wall metabolism in ripening fruit. II. Changes in carbohydrate degrading enzymes in ripening barlett pears. Plant Physiol. 65: 1014–1016.
Arnon D.L., 1949. Copper enzyme in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 24: 1–5.
Bal J.S., P. Singh and S.S. Mann, 1978. Changes in total yellow pigment, protein and total carbohydrates during ripening of ber fruits. Prog. Hortic. 10(1): 73–75.
Blumekrantz N. and G. Asboe-Hansen, 1973. New method for quantitative determination of uronide acids. Analytical Biochem. 54: 484–489.
Cheng G.W. and D.J. Huber, 1996. Alteration in structural polysaccharides during liquifaction of tomato locule tissue. Plant Physiol. 111: 447–457.
Cheng G.W. and D.J. Huber, 1997. Carbohydrate solubilization of tomato locule tissue liquifaction during ripening. Physiol. Plant. 101: 51–58.
Chin L.H., Z.M. Ali and H. Lazan, 1994. Comparative softening of guava fruits. Solubilization and depolymerization of cell wall carbohydrates during ripening. Proc. Malayasian Biochem. Soc. Confer. 19: 147–150.
Clegg K.M., 1956. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1: 40–44.
El-Zoghbi M., 1994. Biochemical changes in some tropical fruits during ripening. Food Chem. 49: 33–37.
Firmin A., 1997. Physico-chemical changes in papaya during storage. Tropical Sci. 37(1): 49–51.
Gray J.E., S. Picton, J. Shabbeer, W. Schuch and D. Gricrson, 1992. Molecular biology of fruit ripening and its manipulation with antisense genes. Plant Mol. Biol. 19: 69–87.
Hobson A.M., 1963. Pectin esterases in normal and abnormal tomato fruit. J. Biochem. 86: 358–361.
Lazan H., M.K. Selamat and Z.M. Ali, 1995. β-galactosidase, polygalacturonase and pectinesterase in different softening and cell wall modification during papaya fruit ripening. Physiol. Plant. 95: 106–112.
Looney N.E. and M.E. Patterson, 1967. Clorophyllase activity in apples and bananas during climacteric phase. Nature 214: 245–246.
Mann S.S., P.N. Singh and R.M. Pandey, 1974. Maturity studies in Dashehari and Langra cvs. Of mango (Mangifera indica L.). Haryana J. Hortic. Sci. 3: 97–105.
Marcelin O., P. William and J.M. Brillouet, 1993. Isolation and characterization of the two main cell types from guava (Psidium guajava L.) pulp. Carb. Res. 240: 233–243.
Martinoida E., M.J. Dalling and P. Matile, 1982. Catabolism of chlorophyll: Demonstration of chloroplast — localized peroxidative and oxidative activities. Z. Pflanzen Physiol. 107: 269–279.
Meel O.P., A.S. Charia, R. Kumar and M.S. Joon, 1991. Physico-chemical changes in the fruits of Sandhura Narnaul ber during growth and development. Haryana J. Hortic. Sci. 20: 65–72.
Mitcham E.J. and R.E. McDonald, 1992. Cell wall modification during ripening of Keitt and Tommy Atkins mango fruits. J. Amer. Soc. Hortic. Sci. 17: 919–924.
Mustaffa R., A. Osman, S. Yusof, and S. Mohamed, 1998. Physico-chemical changes in Cavendish banana (Musa cavendishii L. var. Montel) at different positions within a bunch during development and maturation. J. Sci. Food Agric. 78(2): 201–207.
Nelson N.J., 1944. A photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 153: 375–380.
Pal D.K. and Y. Selvaraj, 1987. Biochemistry of papaya fruit ripening changes in RNA: DNA, protein and enzymes of mitochondrial, carbohydrate, respiratory and phosphate metabolism. J. Hortic. Sci. 62: 117–124.
Pandey M., G.C. Srivastava and N.K. Prasad, 1998. Physiological changes associated with ripening in two mango varieties. Indian J. Plant Physiol. 3:94–96.
Paull R.E. and T. Goo, 1983. Relationship of guava (Psidium guajava L.) fruit detachment force to the stage of fruit development and chemical composition. Hort. Sci. 18: 65–67.
Prabha T.N. and N. Bhagyalakshmi, 1998. Carbohydrate metabolism in ripening banana fruit. Phytochem. 48: 915–919.
Priya Sethu K.M., T.N. Prabha and R.N. Tharanathan, 1996. Post-harvest biochemical changes associated with the softening-phenomenon in Capsicum annuum fruits. Phytochem. 42(4): 961–966.
Richardson C. and G.E. Hobson, 1987. Composition changes in normal and mutant tomato fruit during ripening and storage. J. Sci. Food Agric., 40: 245–252.
Samson J.A., 1986. Tropical Fruits, Second edition. Longman Scientific and Technol, Longman Group UK Ltd.
Sanchez J.A., J.P. Zamorano and R. Alique, 1998. Polygalacturonase, cellulase and invertase activities during cherimoya fruit ripening. J. Hortic. Sci. Biotechnol. 73: 87–92.
Schubret Et. and E.M. Dathe, 1973. Experiments to determine the maturity of apples for storing. Arch. Cartenb. 21: 353–359.
Selvaraj Y. and R. Kumar, 1989. Studies on fruits softening enzymes and polyphenol oxidase activity in ripening mango (Mangifera indica L.) fruit. J. Food Sci. Technol. 26: 218–222.
Sharma R.K., 1996. Physiological and biochemical studies during ripening in ber fruits on tree and in storage. Ph.D. Thesis, CCS Haryana Agric. Univ., Hisar.
Singh A. and M. Singh, 1993. Cell wall degrading enzymes in Orobanche aegyptiaca and its host Brassica campestris. Physiol. Plant. 89: 177–181.
Singh M., K. Dhawan and S. Malhotra, 2000. Carbohydrate metabolism in Tomato (Lycopersicon esculentum L. Mill) fruits during ripening. J. Food Sci. Technol. 37: 222–226.
Singh R. and K. Pradhan, 1980. Forage evaluation. Allied Publishers, New Delhi.
Somogyi M., 1952. Notes on sugar determination. J. Biol. Chem. 195: 19–23.
Theologis A., T.L. Zarembenski, P.W. Oeller, Z. Liang and S. Abel, 1992. Modification of fruit ripening by suppressing gene expression. Plant Physiol. 100: 549–551.
Wellburn A.R., 1994. The spectral determination of chlorophylls a and b as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144: 307–313.
Wilson C.W., 1980. Guava. In: Nagy, S. and Shaw, P.E. (eds.). Tropical and Subtropical Fruits: Composition, Properties and Uses. AVI Publ. Inc., Westport Connecticut.
Yang R.F., T.S. Cheng and R.L. Shewfelt, 1990. The effect of high temperature and ethylene treatment on the ripening of tomatoes. J. Plant Physiol, 136: 368–372.
Yemm E.W. and A.J. Willis, 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57: 508–514.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, N., Dhawan, K., Malhotra, S.P. et al. Compositional and enzymatic changes in guava (Psidium guajava L.) fruits during ripening. Acta Physiol Plant 23, 357–362 (2001). https://doi.org/10.1007/s11738-001-0044-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-001-0044-7