Abstract
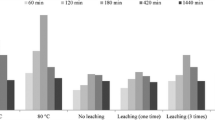
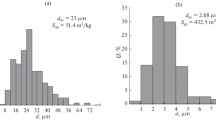
Mn3O4 nanoparticles were prepared by co-precipitation method. The prepared samples had been characterized to find the compositional, structural, and functional properties, by means of EDX, XRD, and FTIR, respectively. The prepared manganese oxide nanoparticles (Mn3O4 NPs) have average crystallite size of 30–35 nm. The effect of different parameters on the uptake of Eu(III) and Gd(III) by Mn3O4 nanoparticles such as pH, initial metal concentration, shaking time, and temperature was examined. The shaking time for both adsorption and desorption was found to be 5 h. The sorption capacities at equilibrium with regards to Eu(III) and Gd(III) were 26.8 and 12.6 mg/g, respectively. Kinetically, the sorption of both elements fitted well to pseudo-second-order model. Sorption equilibrium isotherm obeys more favorably the Langmuir isotherm model. Desorption process of Eu(III) and Gd(III) from Mn3O4 NPs was highly managed using 2.0 M HNO3. A preconcentration factor of 70 and 20 was obtained for Gd and Eu, respectively, using 0.1 g of the Mn3O4 nanoparticles.










Similar content being viewed by others
References
Aghamohammadhasan M, Ghashamsham V, Ghorbani M, Chamsaz M, Masrournia M, Pedramrad T, Akhlaghi H (2017) Preconcentration of gadolinium ion by solidification of floating organic drop microextraction and its determination by UV–Vis Spectrophotometry. Euras J Anal Chem 12(8):1621–1629
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm 77(1):16–23
Al Lafi AG, Al Abdullah J (2015) Cesium and cobalt adsorption on synthetic nano manganese oxide: a two dimensional infra-red correlation spectroscopic investigation. J Mol Struct 1093:13–23
Alizadeh T, Amjadi S (2013) Synthesis of nano-sized Eu3+-imprinted polymer and its application for indirect voltammetric determination of europium. Talanta 106:431–439
Bartolini ME, Pekar J, Chettle DR, McNeill F, Scott A, Sykes J, Moran GR (2003) An investigation of the toxicity of gadolinium based MRI contrast agents using neutron activation analysis. Magn Reson Imaging 21(5):541–544
Bastami TR, Entezari MH (2010) Sono-synthesis of Mn3O4 nanoparticles in different media without additives. Chem Eng J 164(1):261–266
Belkhedkar MR, Ubale AU (2014) Physical properties of nanostructured Mn3O4 thin films synthesized by SILAR method at room temperature for antibacterial application. J Mol Struct 1068:94–100
Burke DM, Morris MA, Holmes JD (2013) Chemical oxidation of mesoporous carbon foams for lead ion adsorption. Sep Purif Technol 104:150–159
Chen X (2015) Modeling of experimental adsorption isotherm data. Information 6(1):14–22
Chen Z, Pan D, Li Z, Jiao Z, Wu M, Shek CH, Lai JK (2014) Recent advances in tin dioxide materials: some developments in thin films, nanowires, and nanorods. Chem Rev 114(15):7442–7486
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using l-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Dhaouadi H, Ghodbane O, Hosni F, Touati F (2012) Mn3O4 nanoparticles: synthesis, characterization and dielectric properties. ISRN Spectrosc 1:1–8
Dubal DP, Dhawale DS, Gujar TP, Lokhande CD (2011) Effect of different modes of electrodeposition on supercapacitive properties of MnO2 thin films. Appl Surf Sci 257(8):3378–3382
Durmus Z, Tomas M, Baykal A, Kavas H, Altincekic TG, Toprak MS (2010) The effect of neutralizing agent on the synthesis and characterization of Mn3O4 nanoparticles. Russ J Inorg Chem 55:1947–1952
El-Sofany E (2008) Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4. J Hazard Mater 153(3):948–954
Elvan H, Ozdes D, Duran C, Sahin D, Tufekci M, Bahadir Z (2013) Separation and preconcentration of copper in environmental samples on Amberlite XAD-8 resin after complexation with a carbothioamide derivative. Quími Nova 36(6):831–835
Fedyunina N, Ossipov I, Seregina M, Bolshov M, Statkus M, Tsysin G (2012) Determination of rare earth elements in rock samples by inductively coupled plasma mass-spectrometry after sorption preconcentration using Pol-DETATA sorbent. Talanta 102:128–131
Fierro V, Torné-Fernández V, Montané D, Celzard A (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater 111(1–3):276–284
Gad HM, El-Sayed AA (2009) Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J Hazard Mater 168(2–3):1070–1081
Gad HMH, Ali MMS, Zaher WF, El-Sofany EA, Abo-El-Enein SA (2014) Application of Olive Stone based activated carbon in the sorption of lanthanum (III) ions from aqueous solution. Arab J Nucl Sci Appl 47(3):67–79
Gad HM, Hamed MM, Eldahab HA, Moustafa ME, El-Reefy SA (2017) Radiation-induced grafting copolymerization of resin onto the surface of silica extracted from rice husk ash for adsorption of gadolinium. J Mol Liq 231:45–55
Galhoum AA, Hassan KM, Desouky OA, Masoud AM, Akashi T, Sakai Y, Guibal E (2017) Aspartic acid grafting on cellulose and chitosan for enhanced Nd(III) sorption. React Funct Polym 113:13–22
Huittinen N, Rabung T, Lützenkirchen J, Mitchell SC, Bickmore BR, Lehto J, Geckeis H (2009) Sorption of Cm(III) and Gd(III) onto gibbsite, α-Al (OH)3: a batch and TRLFS study. J Colloid Interface Sci 332(1):158–164
Kanna M, Wongnawa S, Sherdshoopongse P, Boonsin P (2005) Adsorption behavior of some metal ions on hydrated amorphous titanium dioxide surface. Adsorption 27(5):1018–1026
Karadas C, Kara D, Fisher A (2011) Determination of rare earth elements in seawater by inductively coupled plasma mass spectrometry with off-line column preconcentration using 2,6-diacetylpyridine functionalized Amberlite XAD-4. Anal Chim Acta 689:184–189
Kazakov AG, Aliev RA, Bodrov AY, Priselkova AB, Kalmykov SN (2018) Separation of radioisotopes of terbium from a europium target irradiated by 27 MeV α-particles. Radiochim Acta 106(2):135–140
Kowanga KD, Gatebe E, Mauti GO, Mauti EM (2016) Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu (II) and Pb(II) using defatted Moringa oleifera seed powder. J Phytopharmacol 5(2):71–78
Laraous S, Meniai AH, Bencheikh LM (2005) Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185:483–490
Lei S, Tang K, Fang Z, Sheng J (2007) One-step synthesis of colloidal Mn3O4 and γ-Fe2O 3 nanoparticles at room temperature. J Nanopart Res 9(5):833–840
Li Y, Hu B (2010) Cloud point extraction with/without chelating agent on-line coupled with inductively coupled plasma optical emission spectrometry for the determination of trace rare earth elements in biological samples. J Hazard Mater 174(1–3):534–540
Liang P, Chen X (2005) Preconcentration of rare earth elements on silica gel loaded with 1-phenyl-3-methyl-4-benzoylpyrazol-5-one prior to their determination by ICP-AES. Anal Sci 21(10):1185–1188
Liang P, Liu Y, Guo L (2005) Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim Acta Part B 60:125–129
Liu YG, Ting L, He ZB, Li TT, Hui W, Hu XJ, Yuan HE (2013) Biosorption of copper (II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. T Nonferr Metal Soc 23(6):1804–1814
Liu Y, Wei J, Tian Y, Yan S (2015) The structure–property relationship of manganese oxides: highly efficient removal of methyl orange from aqueous solution. J Mater Chem A 3(37):19000–19010
Mahmoud MA (2015) Kinetics and thermodynamics of aluminum oxide nanopowder as adsorbent for Fe(III) from aqueous solution. J Basic Appl Sci 4(2):142–149
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: kinetic study and equilibrium isotherm analyses. Chemosphere 61(4):492–501
Mallah MH, Shemirani F, Maragheh MG (2008) Use of dispersive liquid-liquid microextraction for simultaneous preconcentration of samarium, europium, gadolinium and dysprosium. J Radioanal Nucl Chem 278:97–102
Mansournia M, Azizi F, Rakhshan N (2015) A novel ammonia-assisted method for the direct synthesis of Mn3O4 nanoparticles at room temperature and their catalytic activity during the rapid degradation of azo dyes. J Phys Chem Solids 80:91–97
Moyer E, McCarthy W (1969) Evaluation of electron spin resonance for quantitative determinations of gadolinium, chromium, iron, copper and manganese. Anal Chim Acta 48:79
Ohashi A, Hashimoto T, Imura H, Ohashi K (2007) Cloud point extraction equilibrium of lanthanum(III), europium(III) and lutetium(III) using di(2-ethylhexyl)phosphoric acid and Triton X-100. Talanta 73:893–898
Othersen J, Maize J, Woolson R, Budisavljevic M (2007) Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transpl 22(11):3179–3185
Özcan A, Özcan AS (2005) Adsorption of Acid Red 57 from aqueous solutions onto surfactant-modified sepiolite. J Hazard Mater 125(1–3):252–259
Person P, Andersson P, Zhang J, Porcelli D (2011) Determination of Nd isotopes in water: a chemical separation technique for extracting Nd from seawater using a chelating resin. Anal Chem 83:1336–1341
Plazinski W, Dziuba J, Rudzinski W (2013) Modeling of sorption kinetics: the pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 19(5):1055–1064
Pyrzynska K (2013) Use of nanomaterials in sample preparation. TrAC, Trends Anal Chem 43:100–108
Rufus AL, Kumar PS, Jeena K, Velmurugan S (2018) Removal of gadolinium, a neutron poison from the moderator system of nuclear reactors. J Hazard Mater 342:77–84
Sadia M, Jan MR, Shah J, Greenway GM (2013) Simultaneous preconcentration and determination of nickel and cobalt using functionalised mesoporous silica spheres by ICP-OES. Int J Environ Anal Chem 93(14):1537–1556
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238
Sayed MA, Helal AI, Abdelwahab SM, Aly HF (2017) Sorption of cesium from aqueous solutions by some Egyptian pottery materials. Appl Clay Sci 139:1–8
Sherin JS, Thomas JK, Suthagar J (2014) Combustion Synthesis and magnetic studies of hausmannite, Mn3O4, nanoparticles. Int J Eng Res Develop 10:34–41
Shizhong C, Mingfa X, Dengbo L, Xilin Z (2007) Carbon nanofibers as solid-phase extraction adsorbent for the preconcentration of trace rare earth elements and their determination by inductively coupled plasma mass spectrometry. Anal Lett 40:2105–2115
Shrihari V, Madhan S, Das A (2005) kinetics of phenol sorption by Raw Agrowastes. Appl Sci 6(1):47–50
Shrividhya T, Ravi G, Mahalingam T, Hayakawa Y (2014) Synthesis and study on structural, morphological and magnetic properties of nanocrystalline manganese oxide. Int J Sci Eng Appl 5:13–16
Singha M, Pal S, Hareendran KN, Roy SB (2014) Poly-hydroxamic acid (PHA) matrix for gadolinium pre-concentration and removal. J Radioanal Nucl Chem 302:961–966
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloids Surf A 272(1–2):89–104
Sun Y, Wang Q, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027
Vázquez-Olmos A, Redón R, Rodríguez-Gattorno G, Mata-Zamora ME, Morales-Leal F, Fernández-Osorio AL, Saniger JM (2005) One-step synthesis of Mn3O4 nanoparticles: structural and magnetic study. J Colloid Interface Sci 291(1):175–180
Zamani HA, Mohammadhosseini M, Haji-Mohammadrezazadeh S, Faridbod F, Ganjali MR, Meghdadi S, Davoodnia A (2012) Gadolinium (III) ion selective sensor using a new synthesized Schiff’s base as a sensing material. Mater Sci Eng, C 32(4):712–717
Zhang P, Zhan Y, Cai B, Hao C, Wang J, Liu C, Chen Q (2010) Shape-controlled synthesis of Mn3O 4 nanocrystals and their catalysis of the degradation of methylene blue. Nano Res 3(4):235–243
Zhang Y, Zhong C, Zhang Q, Chen B, He M, Hu B (2015) Graphene oxide-TiO2 composite as a novel adsorbent for the preconcentration of heavy metals and rare earth elements in environmental samples followed by on-line inductively coupled plasma optical emission spectrometry detection. RSC Adv 5:5996–6005
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sayed, M.A., Helal, A.I., Abdelwahab, S.M. et al. Sorption and possible preconcentration of europium and gadolinium ions from aqueous solutions by Mn3O4 nanoparticles. Chem. Pap. 74, 619–630 (2020). https://doi.org/10.1007/s11696-019-00906-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00906-7