Abstract
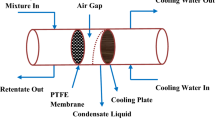
The potential use of air-gap membrane distillation (AGMD) process for the removal of copper sulfate (CuSO4) from aqueous solution was examined. A series of experiments were conducted to investigate the effects of both operation parameters and module parameters, including hot feed temperature(T3), coolant temperature (T1), feed flow rate (F), feed salt concentration (cf), vacuum pressure (P), the rate of hollow fiber membranes and heat-exchange hollow fibers (Nm/Nd) and the length of the modules (L) on permeate water flux (J), gained output ratio (GOR), water productivity (PV), and salt rejection rate (R) in AGMD process. It was found that the performance of AGMD process could be enhanced, but permeate water quality was deteriorated by adding vacuum pressure. Moreover, J declined, but PV and GOR increased as L increased. With the increment of Nm/Nd, J decreased when operation parameters keep constant, PV and GOR showed different variation trends with different T1 values due to the insufficient cold energy. The highest CuSO4 rejection rate exceeded 99.95%, so AGMD process has potential for CuSO4 separation from aqueous solution.










Similar content being viewed by others
References
Ahmad AL, Ooi BS (2010) A study on acid reclamation and copper recovery using low pressure nanofiltration membrane. Chem Eng J 156:257–263. https://doi.org/10.1016/j.cej.2009.10.014
Alkhudhiri A, Hilal N (2017) Air gap membrane distillation: a detailed study of high saline solution. Desalination 403:179–186. https://doi.org/10.1016/j.desal.2016.07.046
Alpatova A, Alsaadi A, Ghaffour N (2018) Boron evaporation in thermally-driven seawater desalination: effect of temperature and operating conditions. J Hazard Mater 351:224–231. https://doi.org/10.1016/j.jhazmat.2018.02.056
Alsaadi AS, Francis L, Maab H, Amy GL, Ghaffour N (2015) Evaluation of air gap membrane distillation process running under sub-atmospheric conditions: experimental and simulation studies. J Membr Sci 489:73–80. https://doi.org/10.1016/j.memsci.2015.04.008
Al-Saydeh SA, El-Naas MH, Zaidi SJ (2017) Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem 56:35–44. https://doi.org/10.1016/j.jiec.2017.07.026
Attia H, Alexander S, Wright CJ, Hilal N (2017) Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 420:318–329. https://doi.org/10.1016/j.desal.2017.07.022
Cheng D, Gong W, Li N (2016) Response surface modeling and optimization of direct contact membrane distillation for water desalination. Desalination 394:108–122. https://doi.org/10.1016/j.desal.2016.04.029
Cheng D, Li N, Zhang J (2018) Modeling and multi-objective optimization of vacuum membrane distillation for enhancement of water productivity and thermal efficiency in desalination. Chem Eng Res Des 132:697–713. https://doi.org/10.1016/j.cherd.2018.02.017
Chou Y-H, Choo K-H, Chen S-S, Yu J-H, Peng C-Y, Li C-W (2018) Copper recovery via polyelectrolyte enhanced ultrafiltration followed by dithionite based chemical reduction: effects of solution pH and polyelectrolyte type. Sep Purif Technol 198:113–120. https://doi.org/10.1016/j.seppur.2017.02.008
Cséfalvay E, Pauer V, Mizsey P (2009) Recovery of copper from process waters by nanofiltration and reverse osmosis. Desalination 240:132–142. https://doi.org/10.1016/j.desal.2007.11.070
Davarnejad R, Panahi P (2016) Cu (II) removal from aqueous wastewaters by adsorption on the modified Henna with Fe3O4 nanoparticles using response surface methodology. Sep Purif Technol 158:286–292. https://doi.org/10.1016/j.seppur.2015.12.018
Dong Y, Liu J, Sui M, Qu Y, Ambuchi JJ, Wang H, Feng Y (2017) A combined microbial desalination cell and electrodialysis system for copper-containing wastewater treatment and high-salinity-water desalination. J Hazard Mater 321:307–315. https://doi.org/10.1016/j.jhazmat.2016.08.034
Duong HC, Chivas AR, Nelemans B, Duke M, Gray S, Cath TY, Nghiem LD (2015) Treatment of RO brine from CSG produced water by spiral-wound air gap membrane distillation—a pilot study. Desalination 366:121–129. https://doi.org/10.1016/j.desal.2014.10.026
Fichera GV, Malagodi M, Cofrancesco P, Weththimuni ML, Guglieri C, Olivi L, Ruffolo S, Licchelli M (2018) Study of the copper effect in iron-gall inks after artificial ageing. Chem Pap 72:1905–1915. https://doi.org/10.1007/s11696-018-0412-z
Geng H, He Q, Wu H, Li P, Zhang C, Chang H (2014a) Experimental study of hollow fiber AGMD modules with energy recovery for high saline water desalination. Desalination 344:55–63. https://doi.org/10.1016/j.desal.2014.03.016
Geng H, Wu H, Li P, He Q (2014b) Study on a new air-gap membrane distillation module for desalination. Desalination 334:29–38. https://doi.org/10.1016/j.desal.2013.11.037
Geng H, Lin L, Li P, Zhang C, Chang H (2015) Study on the heat and mass transfer in AGMD module with latent heat recovery. Desalin Water Treat 57:15276–15284. https://doi.org/10.1080/19443994.2015.1074122
Gryta M (2016) The application of polypropylene membranes for production of fresh water from brines by membrane distillation. Chem Pap 71:775–784. https://doi.org/10.1007/s11696-016-0059-6
Gryta M (2017) The long-term studies of osmotic membrane distillation. Chem Pap 72:99–107. https://doi.org/10.1007/s11696-017-0261-1
Gryta M, Waszak M (2016) Application of vacuum membrane distillation for concentration of organic solutions. Chem Pap 70:737–746. https://doi.org/10.1515/chempap-2016-0002
Guan Y, Li J, Cheng F, Zhao J, Wang X (2015) Influence of salt concentration on DCMD performance for treatment of highly concentrated NaCl, KCl, MgCl2 and MgSO4 solutions. Desalination 355:110–117. https://doi.org/10.1016/j.desal.2014.10.005
He J, Zhang L, Zhang K, Qin Y, Liu L (2015) Concentrating aqueous urea solution by using continuous-effect membrane distillation. Chem Eng Res Des 104:589–604. https://doi.org/10.1016/j.cherd.2015.10.002
Hu H, Li X, Huang P, Zhang Q, Yuan W (2017) Efficient removal of copper from wastewater by using mechanically activated calcium carbonate. J Environ Manag 203:1–7. https://doi.org/10.1016/j.jenvman.2017.07.066
Jia F, Wang J (2017) Separation of cesium ions from aqueous solution by vacuum membrane distillation process. Prog Nucl Energy 98:293–300. https://doi.org/10.1016/j.pnucene.2017.04.008
Jia F, Yin Y, Wang J (2018) Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog Nucl Energy 103:20–27. https://doi.org/10.1016/j.pnucene.2017.11.008
Karakulski K, Gryta M (2016) The application of ultrafiltration for treatment of ships generated oily wastewater. Chem Pap 71:1165–1173. https://doi.org/10.1007/s11696-016-0108-1
Kim Y-D, Thu K, Ghaffour N, Choon Ng K (2013) Performance investigation of a solar-assisted direct contact membrane distillation system. J Memb Sci 427:345–364. https://doi.org/10.1016/j.memsci.2012.10.008
Lawson KW, Lloyd DR (1997) Membrane distillation. J Membr Sci 124:1–25. https://doi.org/10.1016/S0376-7388(96),00236-0
Liu Z, Gao Q, Lu X, Ma Z, Zhang H, Wu C (2017) Experimental study of the optimal vacuum pressure in vacuum assisted air gap membrane distillation process. Desalination 414:63–72. https://doi.org/10.1016/j.desal.2017.03.031
Miyawaki O, Saito A, Matsuo T, Nakamura K (2014) Activity and activity coefficient of water in aqueous solutions and their relationships with solution structure parameters. Biosci Biotechnol Biochem 61:466–469. https://doi.org/10.1271/bbb.61.466
Ntagia E, Rodenas P, ter Heijne A, Buisman CJN, Sleutels THJA (2016) Hydrogen as electron donor for copper removal in bioelectrochemical systems. Int J Hydrog Energy 41:5758–5764. https://doi.org/10.1016/j.ijhydene.2016.02.058
Ntimbani RN, Simate GS, Ndlovu S (2016) Removal of copper ions from dilute synthetic solution using staple ion exchange fibres: dynamic studies. J Environ Chem Eng 4:3143–3150. https://doi.org/10.1016/j.jece.2016.06.023
Ozbey-Unal B, Imer DY, Keskinler B, Koyuncu I (2018) Boron removal from geothermal water by air gap membrane distillation. Desalination 433:141–150. https://doi.org/10.1016/j.desal.2018.01.033
Pal P, Manna AK (2010) Removal of arsenic from contaminated groundwater by solar-driven membrane distillation using three different commercial membranes. Water Res 44:5750–5760. https://doi.org/10.1016/j.watres.2010.05.031
Qiu X, Hu H, Yang J, Wang C, Cheng Z, Ji G (2018) Selective removal of copper from simulated nickel electrolyte by polystyrene-supported 2-aminomethylpyridine chelating resin. Chem Pap 72:2071–2085. https://doi.org/10.1007/s11696-018-0436-4
Torma CZ, Cséfalvay E (2018) Nanofiltration and electrodialysis: alternatives in heavy metal containing high salinity process water treatment. Chem Pap 72:1115–1124. https://doi.org/10.1007/s11696-018-0433-7
Urbina L, Guaresti O, Requies J, Gabilondo N, Eceiza A, Corcuera MA, Retegi A (2018) Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohyd Polym 193:362–372. https://doi.org/10.1016/j.carbpol.2018.04.007
Zoungrana A, Çakmakci M, Zengin İH, İnoğlu Ö, Elcik H (2016) Treatment of metal-plating waste water by modified direct contact membrane distillation. Chem Pap 70:1185–1195. https://doi.org/10.1515/chempap-2016-0066
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, CH., Zhao, YJ., Cheng, L. et al. Removal of copper sulfate from aqueous solution by air-gap membrane distillation process. Chem. Pap. 73, 543–554 (2019). https://doi.org/10.1007/s11696-018-0611-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0611-7