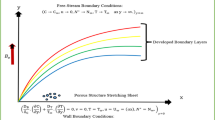
Abstract
A bubble column was investigated in which a swarm of air bubbles was dispersed in aqueous electrolyte (NaCl, MgSO4·7H2O, CaCl2·2H2O, and Na2SO4) solutions. In the present work, study of coalescence inhibition has been targeted by applying gas holdup enhancement and surface tension gradient approaches for aqueous solutions in single and binary mixtures (CaCl2·2H2O + NaCl and Na2SO4 + NaCl) of electrolytes. Transition concentrations of a series of coalescence inhibiting inorganic electrolytes were determined. A qualitative comparison of these electrolytes revealed that strong electrolytes (Na2SO4, and CaCl2·2H2O) yield gas holdup enhancement ≥ 60% whereas moderate electrolytes (NaCl and MgSO4·7H2O) give gas holdup enhancement values ≤ 46%. It has been also found that the values of transition concentration for different electrolytes are of the same order in most of the cases and in line with those reported in the literature. Inhibition of bubble coalescence was also analyzed in terms of the parameter \( C\left( {{\text{d}}\sigma /{\text{d}}C} \right)^{2} \). The large value of the parameter \( ({\text{d}}\sigma /{\text{d}}C)^{2} \) indicates that the electrolyte will inhibit bubble coalescence, and a smaller value indicates moderate effect on bubble coalescence. Surface elasticity values at transition concentration of various electrolytes were also determined. It was found that the surface elasticity values at transition concentration were in the order CaCl2·2H2O > MgSO4·7H2O > Na2SO4 > NaCl. Surface elasticity for binary electrolytes was also estimated at their transition concentrations. The values were found in the order CaCl2·2H2O + NaCl > Na2SO4 + NaCl. Furthermore, analysis of variance was employed to estimate significance of the parameters.
Graphical abstract




















Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- GH:
-
Gas holdup
- MOC:
-
Material of construction
- HM:
-
Homogenous regime
- HT:
-
Heterogenous regime
- A :
-
Hamaker constant (non-retarded) for water, (3.5 × 10−20 J)
- a ± :
-
Mean ion activity coefficient
- a f :
-
Free area of the disc
- c :
-
Force defined by Eq. (2), N
- C trans :
-
Transition concentration (mol/L)
- C :
-
Electrolyte concentration (mol/L)
- ΔC :
-
Change in surface tension of solute (electrolyte)
- H L :
-
Liquid height in bubble column (m)
- H b :
-
Aerated froth height in bubble column (m)
- k :
-
Defined by Eq. (3) (l/m)
- R :
-
Universal gas constant, J/mol K
- r :
-
Bubble radius (m)
- T :
-
Absolute temperature (K)
- U g :
-
Velocity of air (m/s)
- U L :
-
Velocity of liquid (m/s)
- ɛ, ɛ G :
-
Gas holdup in aqueous solution of electrolyte (dimensionless)
- ɛ w :
-
Gas holdup in distilled water (dimensionless)
- υ :
-
Number of ions formed on dissociation (i.e., υ = 2 for most inorganic salt)
- σ, σ el or σ aqueous :
-
Surface tension of electrolyte solutions (mN/m)
- σ w :
-
Surface tension of water (mN/m)
- Δσ :
-
Mean change in surface tension (mN/m)
- dσ/dC :
-
Surface tension gradient
- ρ aqueous :
-
Density of aqueous solution of electrolyte (kg/m3)
- ψ aqueous :
-
Conductivity of aqueous solution of electrolyte (µS/m)
References
Akita K, Yoshida F (1973) Gas holdup and volumetric mass transfer coefficient in bubble columns. Effects of liquid properties, industrial and engineering chemistry process. Des Dev 12(1):76–80. https://doi.org/10.1021/i260045a015
Al Taweel AM, Idhbeaa AO, Ghanem A (2013) Effect of electrolytes on interphase mass transfer in microbubble-sparged airlift reactors. Chem Eng Sci 100:474–485. https://doi.org/10.1016/j.ces.2013.06.013
Bach HF, Pilhofer T (1978) Variation of gas hold-up in bubble columns with physical properties of liquids and operating parameters of columns. Ger Chem Eng 1:270–275
Besagni G, Inzoli F (2016) Bubble size distributions and shapes in annular gap bubble column. Exp Therm Fluid Sci 74:27–48. https://doi.org/10.1016/j.expthermflusci.2015.11.020
Besagni G, Inzoli F (2017a) The effect of electrolyte concentration on counter-current gas-liquid bubble column fluid dynamics: gas holdup, flow regime transition and bubble size distributions. Chem Eng Res Des 118:170–193. https://doi.org/10.1016/j.cherd.2016.12.012
Besagni G, Inzoli F (2017b) The effect of liquid phase properties on bubble column fluid dynamics: gas holdup, flow regime transition, bubble size distributions and shapes, interfacial areas and foaming phenomena. Chem Eng Sci 170:270–296. https://doi.org/10.1016/j.ces.2017.03.043
Besagni G, Di Pasquali A, Gallazzini L, Gottardi E, Colombo LPM, Inzoli F (2017c) The effect of aspect ratio in counter-current gas-liquid bubble columns: experimental results and gas holdup correlations. Int J Multiph Flow 94:53–78. https://doi.org/10.1016/j.ijmultiphaseflow.2017.04.015
Besagni G, Inzoli F, De Guido G, Pellegrini LA (2017d) The dual effect of viscosity on bubble column hydrodynamics. Chem Eng Sci 158:509–538. https://doi.org/10.1016/j.ces.2016.11.003
Besagni G, Gallazzini L, Inzoli F (2018) Effect of gas sparger design on bubble column hydrodynamics using pure and binary liquid phases. Chem Eng Sci 176:116–126. https://doi.org/10.1016/j.ces.2017.10.036
Cents AHG, Jansen DJW, Brilman DWF, Versteeg GF (2005) Influence of small amounts of additives on gas hold-up, bubble size, and interfacial area. Ind Eng Chem Res 44(14):4863–4870. https://doi.org/10.1021/ie049475f
Chan BS, Tsang YH (2005) A theory on bubble-size dependence of the critical electrolyte concentration for inhibition of coalescence. J Colloid Interface Sci 286(1):410–413. https://doi.org/10.1016/j.jcis.2005.01.048
Christenson HK, Yaminsky VV (1995) Solute effects on bubble coalescence. J Phys Chem 99(25):10420. https://doi.org/10.1021/j100025a052
Christenson HK, Bowen RE, Carlton JA, Denne JRM, Lu Y (2008) Electrolytes that show a transition to bubble coalescence inhibition at high concentrations. J Phys Chem C 112(3):794–796. https://doi.org/10.1021/jp075440s
Craig VS (2011) Do hydration forces play a role in thin film drainage and rupture observed in electrolyte solutions? Curr Opin Colloid Interface Sci 16(6):597–600. https://doi.org/10.1016/j.cocis.2011.04.003
Craig VSJ, Ninham BW, Pashley RM (1993a) Effect of electrolytes on bubble coalescence. Nature 364(6435):317–319. https://doi.org/10.1038/364317a0
Craig VSJ, Ninham BW, Pashley RM (1993b) The effect of electrolytes on bubble coalescence in water. J Phys Chem 97(39):10192–10197. https://doi.org/10.1021/j100141a047
Del Castillo LA, Ohnishi S, Horn RG (2011) Inhibition of bubble coalescence: effects of salt concentration and speed of approach. J Colloid Interface Sci 356(1):316–324. https://doi.org/10.1016/j.jcis.2010.12.057
Deschenes LA, Barrett J, Muller LJ, Fourkas JT, Mohanty U (1998) Inhibition of bubble coalescence in aqueous solutions. 1. Electrolytes. J Phys Chem B 102(26):5115–5119. https://doi.org/10.1021/jp980828w
Eissa SH, Schügerl K (1975) Holdup and backmixing investigations in cocurrent and countercurrent bubble columns. Chem Eng Sci 30(10):1251–1256. https://doi.org/10.1016/0009-2509(75)85048-2
Geffcken G (1904) Comparative solubility of gases, etc., in water and in aqueous solutions. Z Phys Chem 49:257–302
Godbole SP, Honath MF, Shah YT (1982) Holdup structure in highly viscous Newtonian and non-Newtonian liquids in bubble columns. Chem Eng Commun 16(1–6):119–134. https://doi.org/10.1080/00986448208911090
Hecht K, Bey O, Ettmüller J, Graefen P, Friehmelt R, Nilles M (2015) Effect of gas density on gas holdup in bubble columns. Chem Ing Tec 87(6):762–772. https://doi.org/10.1002/cite.201500010
Henry CL, Dalton CN, Scruton L, Craig VS (2007) Ion-specific coalescence of bubbles in mixed electrolyte solutions. J Phys Chem C 111(2):1015–1023. https://doi.org/10.1021/jp066400b
Henry CL, Parkinson L, Ralston JR, Craig VS (2008) A mobile gas–water interface in electrolyte solutions. J Phys Chem C 112(39):15094–15097. https://doi.org/10.1021/jp8067969
Jackson AT (1991) Process engineering in biotechnology. Prentice Hall International, UK
Joshi JB, Veera VP, Prasad CV, Phanilumar DV, Deshphande NS, Thakre SS, Thorat BN (1998) Gas holdup structure in bubble column reactors. PINSA 64(4):441–567
Joshi JB, Vitankar VS, Kulkarni AA, Dhotre MT, Ekambara K (2002) Coherent flow structures in bubble column reactors. Chem Eng Sci 57(16):3157–3183. https://doi.org/10.1016/S0009-2509(02)00192-6
Kastanek F, Zahradnik J, Kratochvil J, Cermak J (1984) Modeling of large-scale bubble column reactors for non-ideal gas–liquid systems. Front Chem React Eng 1:330
Kelkar BG, Phulgaonkar SR, Shah YT (1983) The effect of electrolyte solutions on hydrodynamic and backmixing characteristics in bubble columnns. Chem Eng J 27(3):125–133. https://doi.org/10.1016/0300-9467(83)80069-0
Khare AS, Joshi JB (1990) Effect of fine particles on gas hold-up in three-phase sparged reactors. Chem Eng J 44(1):11–25. https://doi.org/10.1016/0300-9467(90)80050-M
Kluytmans JHJ, Van wachem BGM, Kuster BFM, Schouten JC (2001) Gas holdup in a slurry bubble column: influence of electrolyte and carbon particles. Ind Eng Chem Res 40(23):5326–5333. https://doi.org/10.1021/ie001078r
Koide K, Takazawa A, Komura M, Matsunaga H (1984) Gas holdup and volumetric liquid-phase mass transfer coefficient in solid-suspended bubble columns. J Chem Eng Jpn 17(5):459–466
Lee JC, Meyrick B (1970) Gas-liquid interfacial areas in salt solutions in an agitated tank. Trans IChemE 48:T37–T45
Lee LS, Sun SL, Lin CL (2008) Predictions of thermodynamic properties of aqueous single-electrolyte solutions with the two-ionic-parameter activity coefficient model. Fluid Phase Equilib 264(1):45–54. https://doi.org/10.1016/j.fluid.2007.10.015
Lessard RR, Zieminski SA (1971) Bubble coalescence and gas transfer in aqueous electrolytic solutions. Ind Eng Chem Fundam 10(2):260–269. https://doi.org/10.1021/i160038a012
Machon V, Vlcek J, Kurdna V(1977) Gas hold-up in agitated aqueous solutions of strong inorganic salts. In: Proc 2nd Europ conf on mixing, Cambridge, England; (BHRA Fluid Eng), F2–17–F2–34
Majumder SK (2016) Hydrodynamics and transport processes of inverse bubbly flow, 1st edn. Elsevier, Amsterdam
Marrucci G, Nicodemo L (1967) Coalescence of gas bubbles in aqueous solutions of inorganic electrolytes. Chem Eng Sci 22:1257–1265. https://doi.org/10.1016/0009-2509(67)80190-8
Marucci G (1969) Theory of coalescence. Chem Eng Sci 24:975–985. https://doi.org/10.1016/0009-2509(69)87006-5
Millero FJ, Huang F, Laferiere AL (2002) Solubility of oxygen in the major sea salts as a function of concentration and temperature. Mar Chem 78:217–230. https://doi.org/10.1016/S0304-4203(02)00034-8
Mouza AA, Dalakoglou GK, Paras SV (2005) Effect of liquid properties on the performance of bubble column reactors with fine pore spargers. Chem Eng Sci 60(5):1465–1475. https://doi.org/10.1016/j.ces.2004.10.013
Nguyen PT, Hampton MA, Nguyen AV, Birkett GR (2012) The influence of gas velocity, salt type and concentration on transition concentration for bubble coalescence inhibition and gas holdup. Chem Eng Res Des 90(1):33–39. https://doi.org/10.1016/j.cherd.2011.08.015
Olivieri G, Russo ME, Simeone M, Marzocchella A, Salatino P (2011) Effects of viscosity and relaxation time on the hydrodynamics of gas–liquid systems. Chem Eng Sci 66(14):3392–3399. https://doi.org/10.1016/j.ces.2011.01.027
Orvalho S, Ruzicka MC, Drahos J (2009) Bubble column with electrolytes: gas holdup and flow regimes. Ind Eng Chem Res 48(17):8237–8243. https://doi.org/10.1021/ie900263d
Pashley RM, Craig VSJ (1997) Effects of electrolytes on bubble coalescence. Langmuir 13(17):4772–4774. https://doi.org/10.1021/la960034d
Patil VK, Joshi JB, Sharma MM (1984) Sectionalised bubble column: gas hold-up and wall side solid–liquid mass transfer coefficient. Can J Chem Eng 62(2):228–232. https://doi.org/10.1002/cjce.5450620210
Prince MJ, Blanch HW (1990) Transition electrolyte concentrations for bubble coalescence. AIChE J 36:1425–1429. https://doi.org/10.1002/aic.690360915
Ribeiro CP, Mewes D (2007a) The effect of electrolytes on the critical velocity for bubble coalescence. Chem Eng J 126(1):23–33. https://doi.org/10.1016/j.cej.2006.08.029
Ribeiro CP Jr, Mewes D (2007b) The influence of electrolytes on gas hold-up and regime transition in bubble columns. Chem Eng Sci 62(17):4501–4509. https://doi.org/10.1016/j.ces.2007.05.032
Ruzicka MC, Drahos J, Mena PC, Teixeira JA (2003) Effect of viscosity on homogeneous–heterogeneous flow regime transition in bubble columns. Chem Eng J 96(1):15–22. https://doi.org/10.1016/j.cej.2003.08.009
Sagert NH, Quinn MJ (1978) The coalescence of gas bubbles in dilute aqueous solutions. Chem Eng Sci 33:1087–1095. https://doi.org/10.1016/0009-2509(78)85014-3
Sarrafi A, Müller-Steinhagen H, Smith JM, Jamialahmadi M (1999) Gas holdup in homogeneous and heterogeneous gas–liquid bubble column reactors. Can J Chem Eng 77(1):11–21. https://doi.org/10.1002/cjce.5450770104
Sasaki S, Hayashi K, Tomiyama A (2016) Effects of liquid height on gas holdup in air–water bubble column. Exp. Therm Fluid Sci. 72:67–74. https://doi.org/10.1016/j.expthermflusci.2015.10.027
Sasaki S, Uchida K, Hayashi K, Tomiyama A (2017) Effects of column diameter and liquid height on gas holdup in air-water bubble columns. Exp Thermal Fluid Sci 82:359–366. https://doi.org/10.1016/j.expthermflusci.2016.11.032
Sharaf S, Zednikova M, Ruzicka MC, Azzopardi BJ (2016) Global and local hydrodynamics of bubble columns–effect of gas distributor. Chem. Eng. J. 288:489–504. https://doi.org/10.1016/j.cej.2015.11.106
Sujan A (2018) Studies on hydrodynamic and mass transfer parameters in a bubble column. Ph.D. thesis, Malaviya National Institute of Technology, Jaipur
Sujan A, Vyas RK (2017) A review on empirical correlations estimating gas holdup for shear-thinning non-Newtonian fluids in bubble column systems with future perspectives. Rev Chem Eng. https://doi.org/10.1515/revce-2016-0062
Sujan A, Vyas RK, Singh K (2018) Estimation of liquid-side mass transfer coefficient and liquid film thickness in a bubble column using single spherical bubble model. Asia- Pac J Chem Eng 2018:e2178. https://doi.org/10.1002/apj.2178
Syeda SR, Reza MJ (2011) Effect of surface tension gradient on gas hold-up enhancement in aqueous solutions of electrolytes. Chem Eng Res Des 89(12):2552–2559. https://doi.org/10.1016/j.cherd.2011.04.013
Thorat BN, Shevade AV, Bhilegaonkar KN, Aglawe RH, Veera UP, Thakre SS, Joshi JB (1998) Effect of sparger design and height to diameter ratio on fractional gas hold-up in bubble columns. Chem Eng Res Des 76(7):823–834. https://doi.org/10.1205/026387698525577
Tsang YH, Koh YH, Koch DL (2004) Bubble-size dependence of the critical electrolyte concentration for inhibition of coalescence. J Colloid Interface Sci 275(1):290–297. https://doi.org/10.1016/j.jcis.2004.01.026
Vakarelski IU, Manica R, Li EQ, Basheva ES, Chan DY, Thoroddsen ST (2018) Coalescence dynamics of mobile and immobile fluid interfaces. Langmuir. https://doi.org/10.1021/acs.langmuir.7b04106
Wang J, Tan SH, Nguyen AV, Evans GM, Nguyen NT (2016) A microfluidic method for investigating ion-specific bubble coalescence in salt solutions. Langmuir 32(44):11520–11524. https://doi.org/10.1021/acs.langmuir.6b03266
Weissenborn PK, Pugh RJ (1995) Surface tension and bubble coalescence phenomena of aqueous solutions of electrolytes. Langmuir 11:1422–1426. https://doi.org/10.1021/la00005a002
Weissenborn PK, Pugh RJ (1996) Surface tension of aqueous solutions of electrolytes: relationship with ion hydration, oxygen solubility, and bubble coalescence. J Colloid Interface Sci 184(2):550–563. https://doi.org/10.1006/jcis.1996.0651
Wilkinson PM, Spek AP, van Dierendonck LL (1992) Design parameters estimation for scale-up of high-pressure bubble columns. AIChE J 38(4):544–554. https://doi.org/10.1002/aic.690380408
Zahradnik J, Peter R, Kastanek F (1987) The effect of liquid phase properties on gas holdup in bubble column reactors. Collect Czech Chem Commun 52(2):335–347
Zahradnik J, Fialova M, Kastanek F, Green K, Thomas N (1995) The effect of electrolytes on bubble coalescence and gas holdup in bubble column reactors. Chem Eng Res Des 73:341–346
Zahradnik J, Fialova M, Ru M, Drahos J, Kastanek F, Thomas NH (1997) Duality of the gas-liquid flow regimes in bubble column reactors. Chem Eng Sci 52(21–22):3811–3826. https://doi.org/10.1016/S0009-2509(97)00226-1
Zemaitis JF Jr, Clark DM, Rafal M, Scrivner NC (1986) Handbook of aqueous electrolyte thermodynamics. DIPPR and AIChE, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sujan, A., Vyas, R.K. Estimation of transition concentration of aqueous mixtures of single and binary electrolytes for bubble coalescence inhibition. Chem. Pap. 72, 2539–2559 (2018). https://doi.org/10.1007/s11696-018-0470-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0470-2