Abstract
Backgrounds
Optimal pain management in bariatric patients is crucial for early recovery. This study aims to evaluate the effects of magnesium and ketamine combination on morphine consumption after open bariatric surgery (primary outcome), as well as on postoperative pain scores and occurrence of side effects.
Method
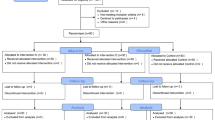
A total of 60 patients undergoing elective open gastric bypass were randomized into 3 groups. All patients received the same general anaesthesia protocol. The magnesium and ketamine group (Mg + K) received an IV bolus of magnesium 50 mg/kg and ketamine 0.2 mg/kg followed by continuous infusion of magnesium (8 mg/kg/h) and ketamine (0.15 mg/kg/h) until extubation. The ketamine group (K) received the same bolus and infusion of ketamine, together with a bolus and continuous infusion of normal saline. The placebo group (P) received normal saline. All patients received 48 h of paracetamol 1 g IV q6h and morphine sulphate 0.1 mg/kg subcutaneous q6h PRN. Morphine consumption, VAS pain scores and occurrence of side effects were recorded for 48 h postoperatively.
Results
Patients in group (Mg + K) (2.4 ± 2.62 mg) and in group (K) (2.8 ± 2.66 mg) had significantly lower morphine consumption in the PACU compared with the patients in group (P) (4.85 ± 4.51 mg) (p = 0.045). Patients in group (Mg + K) consumed significantly less morphine the first 24 postoperative hours, with a relative reduction of 87% and 21% compared with group (K) and group (P) respectively (p = 0.028). However, this difference was not observed at 48 h. No significant difference was shown between the three groups in terms of nausea and vomiting, time to extubation or excessive sedation.
Conclusion
The association of magnesium and ketamine bolus followed by infusion in open bariatric surgery appears to be safe and decreases morphine requirements in the first 24 h compared with both ketamine alone and placebo.

Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Fernandez AZ, Demaria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–703.
Sudré ECM, de Batista PR, Castiglia YMM. Longer immediate recovery time after anesthesia increases risk of respiratory complications after laparotomy for bariatric surgery: a randomized clinical trial and a cohort study. Obes Surg. 2015;25:2205–12.
Egan TD, Huizinga B, Gupta SK, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. 1998;89:562–73.
Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiol J Am Soc Anesthesiol. 2000;93:409–17.
Crawford M, Hickey C, Zaarour C, et al. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662–7.
Lee M, Silverman S, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–61.
Ramasubbu C, Gupta A. Pharmacological treatment of opioid-induced hyperalgesia: a review of the evidence. J Pain Palliat Care Pharmacother. 2011;25:219–30.
Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–55.
Song JW, Lee Y-W, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg. 2011;113:390–7.
Boenigk K, Echevarria GC, Nisimov E, et al. Low-dose ketamine infusion reduces postoperative hydromorphone requirements in opioid-tolerant patients following spinal fusion: a randomised controlled trial. Eur J Anaesthesiol. 2019;36:8–15.
Nielsen RV, Fomsgaard JS, Siegel H, et al. Intraoperative ketamine reduces immediate postoperative opioid consumption after spinal fusion surgery in chronic pain patients with opioid dependency: a randomized, blinded trial. Pain. 2017;158:463–70.
Lahtinen P, Kokki H, Hakala T, et al. S(+)-ketamine as an analgesic adjunct reduces opioid consumption after cardiac surgery. Anesth Analg. 2004:1295–301.
Dahmani S, Michelet D, Abback P-S, et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth. 2011;21:636–52.
Elia N, Tramèr MR. Ketamine and postoperative pain – a quantitative systematic review of randomised trials. Pain. 2005;113:61–70.
Iseri LT, French JH. Magnesium: nature’s physiologic calcium blocker. Am Heart J. 1984;108:188–93.
Mebazaa MS, Ouerghi S, Frikha N, et al. Is magnesium sulfate by the intrathecal route efficient and safe? Ann Fr Anesth Reanim. 2011;30:47–50.
Tramer MR, Schneider J, Marti R-A, et al. Role of magnesium sulfate in postoperative analgesia. Anesthesiol J Am Soc Anesthesiol. 1996;84:340–7.
Jabbour HJ, Naccache NM, Jawish RJ, et al. Ketamine and magnesium association reduces morphine consumption after scoliosis surgery: prospective randomised double-blind study. Acta Anaesthesiol Scand. 2014;58:572–9.
Guo B-L, Lin Y, Hu W, et al. Effects of systemic magnesium on post-operative analgesia: is the current evidence strong enough? Pain Physician. 2015;18:405–18.
De Oliveira GS, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:178–90.
Albrecht E, Kirkham KR, Liu SS, et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia. 2013;68:79–90.
Savic Vujovic KR, Vuckovic S, Srebro D, et al. A synergistic interaction between magnesium sulphate and ketamine on the inhibition of acute nociception in rats. Eur Rev Med Pharmacol Sci. 2015;19:2503–9.
Liu H-T, Hollmann M, Liu W-H, et al. Modulation of NMDA receptor function by ketamine and magnesium: part I. Anesth Analg. 2001;92:1173–81.
Lemmens HJM, Brodsky JB, Bernstein DP. Estimating ideal body weight – a new formula. Obes Surg. 2005;15:1082–3.
Dang JT, Szeto VG, Elnahas A, et al. Canadian consensus statement: enhanced recovery after surgery in bariatric surgery. Surg Endosc. 2019;
Nagappa M, Weingarten TN, Montandon G, et al. Opioids, respiratory depression, and sleep-disordered breathing. Best Pract Res Clin Anaesthesiol. 2017;31:469–85.
de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31:499–504.
American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on acute pain management. Anesthesiology. 2012;116:248–73.
Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev [Internet]. 2018 [cited 2019 Jun 2]; Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012033.pub4/full
Peltoniemi MA, Hagelberg NM, Olkkola KT, et al. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55:1059–77.
Levaux C, Bonhomme V, Dewandre PY, et al. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia. 2003;58:131–5.
O’Flaherty JE, Lin CX. Does ketamine or magnesium affect posttonsillectomy pain in children? Pediatr Anesth. 2003;13:413–21.
Wacker WE, Parisi AF. Magnesium metabolism. N Engl J Med. 1968;278:712–7.
Ryu J-H, Kang M-H, Park K-S, et al. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br J Anaesth. 2008;100:397–403.
Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:456–66.
Koinig H. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87:206–10.
Dubé L, Granry J-C. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: a review. Can J Anaesth J Can Anesth. 2003;50:732–46.
Fuchs-Buder T, Wilder-Smith OHG, Borgeat A, et al. Interaction of magnesium sulphate with vecuronium-induced neuromuscular block. Br J Anaesth. 1995;74:405–9.
Singh PM, Borle A, McGavin J, et al. Comparison of the recovery profile between desflurane and sevoflurane in patients undergoing bariatric surgery-a meta-analysis of randomized controlled trials. Obes Surg. 2017;27:3031–9.
Schulz-Stübner S, Wettmann G, Reyle-Hahn SM, et al. Magnesium as part of balanced general anaesthesia with propofol, remifentanil and mivacurium: a double-blind, randomized prospective study in 50 patients. Eur J Anaesthesiol. 2001;18:723–9.
Carr BM, Lyon JA, Romeiser J, et al. Laparoscopic versus open surgery: a systematic review evaluating Cochrane systematic reviews. Surg Endosc. 2018:1–17.
Jackson SA, Laurence AS, Hill JC. Does post-laparoscopy pain relate to residual carbon dioxide? Anaesthesia. 1996;51:485–7.
Joris J, Thiry E, Paris P, et al. Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg. 1995;81:379–84.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2065–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jabbour, H., Jabbour, K., Abi Lutfallah, A. et al. Magnesium and Ketamine Reduce Early Morphine Consumption After Open Bariatric Surgery: a Prospective Randomized Double-Blind Study. OBES SURG 30, 1452–1458 (2020). https://doi.org/10.1007/s11695-019-04317-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04317-1