Abstract
Background
The effect of probiotic supplements among subjects undergoing bariatric surgery indicates conflicting results. Moreover, whether these effects remain after ceasing the treatment remained to be elucidated. This study was conducted to assess the effect of probiotic supplements on blood markers of endotoxin (lipopolysaccharides-binding protein: LBP), inflammation and lipid peroxidation (malondialdehyde: MDA) in patients with morbid obesity undergoing the one-anastomosis gastric bypass (OAGB).
Methods
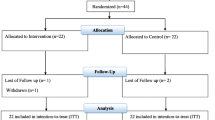
This study is a placebo-controlled, double-blind, and randomized clinical trial and 9 months of additional follow-up. Forty-six morbid obese patients undergoing OAGB were randomized to 4 months of probiotic or placebo supplements. Anthropometric indices and blood concentration of LBP, inflammatory markers, MDA, vitamin D3, and B12 were measured at 0, 4, and 13 months of study.
Results
Probiotic supplements could improve serum LBP (P = 0.039), TNF-α (P = 0.005), vitamin B12 (P = 0.03), vitamin D3 (P = 0.001), and weight loss (P = 0.01) at month 4 in comparison to placebo; however, only serum MDA concentrations decreased significantly in the probiotic group compared with those in the placebo group (P = 0.013) at the end of follow-up period.
Discussion
It was observed that 4 months probiotic supplementation compared with placebo prohibited an elevation in the LBP levels and improved serum TNF-α and 25-OH vitamin D3 concentrations and weight loss in patients undergoing the OAGB surgery. However, these effects did not persist 9 months after the cessation of the treatment. Further investigations are required to find how long supplementation and which dosage of it can benefit body status for the long-term.
Trial Registration
This study has been registered at Clinicaltrial.gov with registration number NCT02708589.




Similar content being viewed by others
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45.
Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–44.
Novitsky TJ. Limitations of the Limulus amebocyte lysate test in demonstrating circulating lipopolysaccharides. Ann N Y Acad Sci. 1998;851(1):416–21.
Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8(3):946–52.
Pussinen PJ, Havulinna AS, Lehto M, et al. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34(2):392–7.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–37.
Mahawar KK, Jennings N, Brown J, et al. “Mini” gastric bypass: systematic review of a controversial procedure. Obes Surg. 2013;23(11):1890–8.
Chang S-H, Stoll CRT, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87.
van Dielen FM, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89(8):4062–8.
Clemente-Postigo M, Roca-Rodriguez MM, Camargo A, et al. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis. 2015;11(4):933–9.
Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94(2):450–8.
Prior SL, Barry JD, Caplin S, et al. Temporal changes in plasma markers of oxidative stress following laparoscopic sleeve gastrectomy in subjects with impaired glucose regulation. Surg Obes Relat Dis. 2017;13(2):162–8.
Monzo-Beltran L, Vazquez-Tarragón A, Cerdà C, et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017;12:389–402.
Savassi-Rocha AL, Diniz MTC, Vilela EG, et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes Surg. 2014;24(2):184–90.
Woodard GA, Encarnacion B, Downey JR, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13(7):1198–204.
Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial–host metabolic cross-talk. Gut. 2011;60(9):1214–23.
Matsumoto S, Hara T, Hori T, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140(3):417–26.
Naito E, Yoshida Y, Makino K, et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110(3):650–7.
Beserra BT, Fernandes R, do Rosario VA, et al. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr. 2015;34(5):845–58.
Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54(7):938–56.
Borgeraas H, Johnson LK, Skattebu J, et al. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018;19(2):219–32.
LeBlanc JG, Laiño JE, del Valle MJ, et al. B-group vitamin production by lactic acid bacteria—current knowledge and potential applications. J Appl Microbiol. 2011;111(6):1297–309.
Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D3: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(7):2944–51.
Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes. 2018;42(2):147–55.
Fernandes R, Beserra BTS, Mocellin MC, et al. Effects of prebiotic and synbiotic supplementation on inflammatory markers and anthropometric indices after Roux-en-Y gastric bypass: a randomized, triple-blind, placebo-controlled pilot study. J Clin Gastroenterol. 2016;50(3):208–17.
Karbaschian Z, Mokhtari Z, Pazouki A, et al. Probiotic supplementation in morbid obese patients undergoing one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB) surgery: a randomized, double-blind, placebo-controlled, clinical trial. Obes Surg. 2018:1–12.
National Health, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. Bethesda; 2000.
Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21(S1)
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17(5):565–8.
Mofidi F, Poustchi H, Yari Z, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117(5):662–8.
Furet J-P, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Isolauri E, Sütas Y, Kankaanpää P, et al. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(2):444s–50s.
Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr. 2017;8(2):240–52.
Yang P-J, Lee WJ, Tseng PH, et al. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis. 2014;10(6):1182–7.
Trøseid M, Nestvold TK, Rudi K, et al. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36(11):3627–32.
Miller GD, Nicklas BJ, Fernandez A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2011;7(5):618–24.
João Cabrera E, Valezi AC, Delfino VD, et al. Reduction in plasma levels of inflammatory and oxidative stress indicators after Roux-en-Y gastric bypass. Obes Surg. 2010;20(1):42–9.
Sabate J-M, Coupaye M, Ledoux S, et al. Consequences of small intestinal bacterial overgrowth in obese patients before and after bariatric surgery. Obes Surg. 2017;27(3):599–605.
Vitetta L, Coulson S, Thomsen M, et al. Probiotics, D-lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017;8(4):311–22.
Carswell KA, Belgaumkar AP, Amiel SA, et al. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes Surg. 2016;26(4):843–55.
da Silva VR, Moreira EA, Wilhelm-Filho D, et al. Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. Eur J Clin Nutr. 2012;66(8):891–9.
Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29(4):591–6.
Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metab Clin Exp. 2016;65(4):574–85.
Lin E, Armstrong-Moore D, Liang Z, et al. Contribution of adipose tissue to plasma 25-hydroxyvitamin D3 concentrations during weight loss following gastric bypass surgery. Obesity. 2011;19(3):588–94.
James H, Lorentz P, Collazo-Clavell ML. Patient-reported adherence to empiric vitamin/mineral supplementation and related nutrient deficiencies after Roux-en-Y gastric bypass. Obes Surg. 2016;26(11):2661–6.
Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569–75.
Acknowledgements
The authors thank all participants and the staffs of Rasoul Hospital, without whom this study was impossible. This study is supported financially by the Shahid Beheshti University of Medical Sciences with grant number: SBMU/6010.
Funding
This study was supported by a grant from the Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Z M and A H conceptualized and designed the study and wrote the manuscript; Z M and Z K collected data; A P, A K, and P M provided the study administration works. A H interpreted the data, provided professional comments, and critically revised the manuscript for intellectual content and data accuracy.
All authors had access to the study data and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study. All patients signed the informed consent form.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute, Tehran, Iran.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mokhtari, Z., Karbaschian, Z., Pazouki, A. et al. The Effects of Probiotic Supplements on Blood Markers of Endotoxin and Lipid Peroxidation in Patients Undergoing Gastric Bypass Surgery; a Randomized, Double-Blind, Placebo-Controlled, Clinical Trial with 13 Months Follow-Up. OBES SURG 29, 1248–1258 (2019). https://doi.org/10.1007/s11695-018-03667-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-03667-6