Abstract
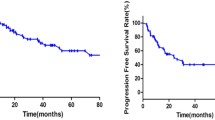
Factors associated with complete and durable remissions after anti-CD19 chimeric antigen receptor T (CAR-T) cell immunotherapy for relapsed or refractory non-Hodgkin lymphoma (r/r NHL) have not been well characterized. In this study, we found that the different sites of extranodal involvement may affect response, overall survival (OS), and progression-free survival (PFS) in patients with r/r NHL treated with anti-CD19 CAR-T cells. In a cohort of 32 treated patients, 12 (37.5%) and 8 (25%) patients exhibited soft tissue lymphoma and bone marrow (BM) infiltrations, respectively, and 13 (41%) patients exhibited infiltration at other sites. The factors that may affect prognosis were identified through multivariable analysis. As an independent risk factor, soft tissue infiltration was the only factor significantly correlated with adverse prognosis (P < 0.05), whereas other factors did not reach statistical significance. Furthermore, the site of extranodal tumor infiltration significantly and negatively affected OS and PFS in patients with r/r NHL treated with anti-CD19 CAR-T cell therapy. PFS and OS in patients with BM involvement were not significantly different from those of patients with lymph node involvement alone. Thus, anti-CD19 CAR-T cell therapy may improve the prognosis of patients with BM infiltration.
Similar content being viewed by others
References
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20(1): 31–42
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377(26): 2531–2544
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017; 35(16): 1803–1813
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377(26): 2545–2554
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126(6): 2123–2138
Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, Mehta B, Zhang H, Dakhova O, Carrum G, Kamble RT, Gee AP, Mei Z, Wu MF, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Savoldo B, Dotti G. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol Ther 2018; 26(12): 2727–2737
Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018; 15(1): 31–46
Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, Li D, Cherian S, Chen X, Pender BS, Hawkins RM, Vakil A, Steinmetz RN, Acharya UH, Cassaday RD, Chapuis AG, Dhawale TM, Hendrie PC, Kiem HP, Lynch RC, Ramos J, Shadman M, Till BG, Riddell SR, Maloney DG, Turtle CJ. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019; 133(17): 1876–1887
Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. Understanding and managing large B cell lymphoma relapses after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant 2019; 25(11): e344–e351
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329(14): 987–994
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32(27): 3059–3068
Del Gobbo A, Fiori S, Ercoli G, Di Bernardo A, Parafioriti A, Fabris S, Iurlo A, Neri A, Bosari S, Gianelli U. Primary soft tissue lymphomas: description of seven cases and review of the literature. Pathol Oncol Res 2017; 23(2): 281–286
Derenzini E, Casadei B, Pellegrini C, Argnani L, Pileri S, Zinzani PL. Non-Hodgkin lymphomas presenting as soft tissue masses: a single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk 2013; 13(3): 258–265
Suárez AL, Pulitzer M, Horwitz S, Moskowitz A, Querfeld C, Myskowski PL. Primary cutaneous B-cell lymphomas: part I. Clinical features, diagnosis, and classification. J Am Acad Dermatol 2013; 69(3): 329.e1–329.e13
Yao Z, Deng L, Xu-Monette ZY, Manyam GC, Jain P, Tzankov A, Visco C, Bhagat G, Wang J, Dybkaer K, Tam W, Hsi ED, van Krieken JH, Ponzoni M, Ferreri AJM, Moller MB, Winter JN, Piris MA, Fayad L, Liu Y, Song Y, Orlowski RZ, Kantarjian H, Medeiros LJ, Li Y, Cortes J, Young KH. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia 2018; 32(2): 353–363
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119(12): 2709–2720
Acknowledgements
This study was supported by grants from the Municipal Hospital Frontier Technology Joint Project of Shanghai City (No. SHDC12015108) and the National Natural Science Foundation of China (Nos. 81830004 and 31301118).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Lili Zhou, Ping Li, Shiguang Ye, Xiaochen Tang, Junbang Wang, Jie Liu, and Aibin Liang declare no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Zhou, L., Li, P., Ye, S. et al. Different sites of extranodal involvement may affect the survival of patients with relapsed or refractory non-Hodgkin lymphoma after chimeric antigen receptor T cell therapy. Front. Med. 14, 786–791 (2020). https://doi.org/10.1007/s11684-020-0751-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-020-0751-3