Abstract
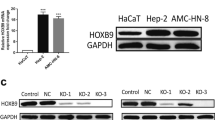
Disabled homolog 2 (DAB2) is frequently deleted or epigenetically silenced in many human cancer cells. Therefore, DAB2 has always been regarded as a tumor suppressor gene. However, the role of DAB2 in tumor progression and metastasis remains unclear. In this study, DAB2 expression was upregulated along with human prostate cancer (PCa) progression. DAB2 overexpression or knockdown effects in LNCaP and PC3 cell lines were verified to address the biological functions of DAB2 in PCa progression and metastasis. LNCaP and PC3 cell lines were generated from human PCa cells with low and high metastatic potentials, respectively. The results showed that DAB2 shRNA knockdown can inhibit the migratory and invasive abilities of PC3 cells, as well as the tumorigenicity, whereas DAB2 overexpression enhanced LNCaP cell migration and invasion. Further investigation showed that DAB2 regulated the cell migration associated genes in PC3 cells, and the differential DAB2 expression between LNCaP and PC3 cells was partly regulated by histone 4 acetylation. Therefore, DAB2 may play an important role in PCa progression and metastasis.
Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69–90
Jilg CA, Ketscher A, Metzger E, Hummel B, Willmann D, Rüsseler V, Drendel V, Imhof A, Jung M, Franz H, Hölz S, Krönig M, Müller JM, Schüle R. PRK1/PKN1 controls migration and metastasis of androgen-independent prostate cancer cells. Oncotarget 2014; 5(24): 12646–12664
Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem 2005; 280(43): 36529–36540
Xie S, Xie Y, Zhang Y, Huang Q. Effects of miR-145 on the migration and invasion of prostate cancer PC3 cells by targeting DAB2. Hereditas (Beijing) (Yi Chuan) 2014; 36(1): 50–57 (in Chinese)
Mok SC, Wong KK, Chan RK, Lau CC, Tsao SW, Knapp RC, Berkowitz RS. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol 1994; 52(2): 247–252
Fu L, Rab A, Tang LP, Rowe SM, Bebok Z, Collawn JF. Dab2 is a key regulator of endocytosis and post-endocytic trafficking of the cystic fibrosis transmembrane conductance regulator. Biochem J 2012; 441(2): 633–643
Moore R, Cai KQ, Tao W, Smith ER, Xu XX. Differential requirement for Dab2 in the development of embryonic and extra-embryonic tissues. BMC Dev Biol 2013; 13(1): 39
Jiang Y, Luo W, Howe PH. Dab2 stabilizes Axin and attenuates Wnt/β-catenin signaling by preventing protein phosphatase 1 (PP1)-Axin interactions. Oncogene 2009; 28(33): 2999–3007
Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-β-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 2010; 12(3): 286–293
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139(5): 871–890
Zhang Z, Chen Y, Tang J, Xie X. Frequent loss expression of dab2 and promotor hypermethylation in human cancers: a meta-analysis and systematic review. Pak J Med Sci 2014; 30(2): 432–437
Mok SC, Chan WY, Wong KK, Cheung KK, Lau CC, Ng SW, Baldini A, Colitti CV, Rock CO, Berkowitz RS. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene 1998; 16(18): 2381–2387
Sheng Z, Sun W, Smith E, Cohen C, Sheng Z, Xu XX. Restoration of positioning control following disabled-2 expression in ovarian and breast tumor cells. Oncogene 2000; 19(42): 4847–4854
Chao A, Lin CY, Lee YS, Tsai CL, Wei PC, Hsueh S, Wu TI, Tsai CN, Wang CJ, Chao AS, Wang TH, Lai CH. Regulation of ovarian cancer progression by microRNA-187 through targeting disabled homolog-2. Oncogene 2012; 31(6): 764–775
Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, Cooper JA. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol 2009; 186(1): 99–111
Chetrit D, Ziv N, Ehrlich M. Dab2 regulates clathrin assembly and cell spreading. Biochem J 2009; 418(3): 701–715
Orlandini M, Nucciotti S, Galvagni F, Bardelli M, Rocchigiani M, Petraglia F, Oliviero S. Morphogenesis of human endothelial cells is inhibited by DAB2 via Src. FEBS Lett 2008; 582(17): 2542–2548
Zhang Y, Xie S, Zhou Y, Xie Y, Liu P, Sun M, Xiao H, Jin Y, Sun X, Chen Z, Huang Q, Chen S. H3K36 histone methyltransferase Setd2 is required for murine embryonic stem cell differentiation toward endoderm. Cell Reports 2014; 8(6): 1989–2002
Liu Y, Song N, Ren K, Meng S, Xie Y, Long Q, Chen X, Zhao X. Expression loss and revivification of RhoB gene in ovary carcinoma carcinogenesis and development. PLoS ONE 2013; 8(11): e78417
Hanker LC, Karn T, Holtrich U, Graeser M, Becker S, Reinhard J, Ruckhäberle E, Gevensleben H, Rody A. Prognostic impact of fascin-1 (FSCN1) in epithelial ovarian cancer. Anticancer Res 2013; 33(2): 371–377
Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T, Seki N. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol 2011; 38(4): 1093–1101
Fu H, Wen JF, Hu ZL, Luo GQ, Ren HZ. Knockdown of fascin1 expression suppresses the proliferation and metastasis of gastric cancer cells. Pathology 2009; 41(7): 655–660
Murray MY, Birkland TP, Howe JD, Rowan AD, Fidock M, Parks WC, Gavrilovic J. Macrophage migration and invasion is regulated by MMP10 expression. PLoS ONE 2013; 8(5): e63555
Xie XM, Zhang ZY, Yang LH, Yang DL, Tang N, Zhao HY, Xu HT, Li QC, Wang EH. Aberrant hypermethylation and reduced expression of disabled-2 promote the development of lung cancers. Int J Oncol 2013; 43(5): 1636–1642
Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT). J Biol Chem 2005; 280(17): 17540–17548
Smith ER, Capo-chichi CD, He J, Smedberg JL, Yang DH, Prowse AH, Godwin AK, Hamilton TC, Xu XX. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem 2001; 276(50): 47303–47310
Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2001; 2(2): 111–123
Xu XX, Yi T, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene 1998; 16(12): 1561–1569
Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor β (TGFβ)-stimulated fibronectin synthesis through TGFβ-activated kinase 1 and activation of the JNK pathway. J Biol Chem 2005; 280(27): 25920–25927
Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 2004; 117(20): 4619–4628
Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J 2001; 20(11): 2789–2801
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xie, Y., Zhang, Y., Jiang, L. et al. Disabled homolog 2 is required for migration and invasion of prostate cancer cells. Front. Med. 9, 312–321 (2015). https://doi.org/10.1007/s11684-015-0401-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-015-0401-3