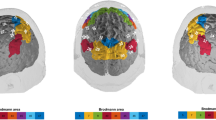
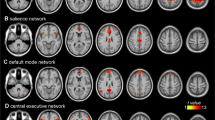
Abstract
The carotenoids lutein (L) and zeaxanthin (Z) accumulate in retinal regions of the eye and have long been shown to benefit visual health. A growing literature suggests cognitive benefits as well, particularly in older adults. The present randomized controlled trial sought to investigate the effects of L and Z on brain function using resting state functional magnetic resonance imaging (fMRI). It was hypothesized that L and Z supplementation would (1) improve intra-network integrity of default mode network (DMN) and (2) reduce inter-network connectivity between DMN and other resting state networks. 48 community-dwelling older adults (mean age = 72 years) were randomly assigned to receive a daily L (10 mg) and Z (2 mg) supplement or a placebo for 1 year. Resting state fMRI data were acquired at baseline and post-intervention. A dictionary learning and sparse coding computational framework, based on machine learning principles, was used to investigate intervention-related changes in functional connectivity. DMN integrity was evaluated by calculating spatial overlap rate with a well-established DMN template provided in the neuroscience literature. Inter-network connectivity was evaluated via time series correlations between DMN and nine other resting state networks. Contrary to expectation, results indicated that L and Z significantly increased rather than decreased inter-network connectivity (Cohen’s d = 0.89). A significant intra-network effect on DMN integrity was not observed. Rather than restoring what has been described in the available literature as a “youth-like” pattern of intrinsic brain activity, L and Z may facilitate the aging brain’s capacity for compensation by enhancing integration between networks that tend to be functionally segregated earlier in the lifespan.

Similar content being viewed by others
References
Andrews-Hanna, J. R., Snyder, A. Z., Vincent, J. L., Lustig, C., Head, D., Raichle, M. E., & Buckner, R. L. (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935.
Baldassarre, A., & Corbetta, M. (2015). Resting state network changes in aging and cognitive decline. Hearing, Balance and Communication, 13(2), 58–64. https://doi.org/10.3109/21695717.2015.1022986.
Baltes, P. B., & Lindenberger, U. (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging, 12(1), 12–21.
Beatty, S., Nolan, J., Kavanagh, H., & O’Donovan, O. (2004). Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Archives of Biochemistry and Biophysics, 430(1), 70–76. https://doi.org/10.1016/j.abb.2004.03.015.
Bernstein, P. S., Li, B., Vachali, P. P., Gorusupudi, A., Shyam, R., Henriksen, B. S., & Nolan, J. M. (2016). Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in Retinal and Eye Research, 50, 34–66.
Bertram, J. S. (1999). Carotenoids and gene regulation. Nutrition Reviews, 57(6), 182–191. https://doi.org/10.1111/j.1753-4887.1999.tb06941.x.
Betzel, R. F., Byrge, L., He, Y., Goñi, J., Zuo, X. N., & Sporns, O. (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102, 345–357.
Binder, J. R., Frost, J. A., Hammeke, T. A., Bellgowan, P. S. F., Rao, S. M., & Cox, R. W. (1999). Conceptual processing during the conscious resting state: A functional MRI study. Journal of Cognitive Neuroscience, 11(1), 80–93.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Boespflug, E. L., McNamara, R. K., Eliassen, J. C., Schidler, M. D., & Krikorian, R. (2016). Fish oil supplementation increases event-related posterior cingulate activation in older adults with subjective memory impairment. The Journal of Nutrition, Health & Aging, 20(2), 161–169. https://doi.org/10.1007/s12603-015-0609-6.
Bokov, A., Chaudhuri, A., & Richardson, A. (2004). The role of oxidative damage and stress in aging. Mechanisms of Ageing and Development, 125(10), 811–826. https://doi.org/10.1016/j.mad.2004.07.009.
Bone, R. A., Landrum, J. T., & Tarsis, S. L. (1985). Preliminary identification of the human macular pigment. Vision Research, 25(11), 1531–1535.
Bookheimer, S. Y., Renner, B. A., Ekstrom, A., Li, Z., Henning, S. M., Brown, J. A., … Small, G. W. (2013). Pomegranate juice augments memory and fMRI activity in middle-aged and older adults with mild memory complaints. Evidence-Based Complementary and Alternative Medicine, 2013, 1, 14 https://doi.org/10.1155/2013/946298.
Bovier, E. R., Renzi, L. M., & Hammond, B. R. (2014). A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS One, 9(9), e108178. https://doi.org/10.1371/journal.pone.0108178.
Brickman, A. M., Khan, U. A., Provenzano, F. A., Yeung, L. K., Suzuki, W., Schroeter, H., Wall, M., Sloan, R. P., & Small, S. A. (2014). Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nature Neuroscience, 17(12), 1798–1803. https://doi.org/10.1038/nn.3850.
Britton, G., Liaaen-Jensen, S., & Pfander, H. (2004). Carotenoids: Handbook. Basel: Springer.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124(1), 1–38. https://doi.org/10.1196/annals.1440.011.
Butterfield, D. A., Bader Lange, M. L., & Sultana, R. (2010). Involvements of the lipid. peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochimica et Biophysica Acta, 1801(8), 924–929. https://doi.org/10.1016/j.bbalip.2010.02.005.
Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100.
Cabeza, R., Daselaar, S. M., Dolcos, F., Prince, S. E., Budde, M., & Nyberg, L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex, 14(4), 364–375.
Cao, M., Wang, J. H., Dai, Z. J., Cao, X. Y., Jiang, L. L., Fan, F. M., ... Milham, M. P. (2014). Topological organization of the human brain functional connectome across the lifespan. Developmental Cognitive Neuroscience, 7, 76–93.
Carp, J., Gmeindl, L., & Reuter-Lorenz, P. A. (2010). Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Frontiers in Human Neuroscience, 4, 217.
Carp, J., Park, J., Hebrank, A., Park, D. C., & Polk, T. A. (2011a). Age-related neural dedifferentiation in the motor system. PLoS One, 6(12), e29411. https://doi.org/10.1371/journal.pone.0029411.
Carp, J., Park, J., Polk, T. A., & Park, D. C. (2011b). Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage, 56(2), 736–743.
Cordes, D., Haughton, V. M., Arfanakis, K., Wendt, G. J., Turski, P. A., Moritz, C. H., ... Meyerand, M. E. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology, 21(9), 1636–1644.
Craft, N. E., Haitema, T. B., Garnett, K. M., Fitch, K. A., & Dorey, C. K. (2004). Carotenoid, tocopherol, and retinol concentrations in elderly human brain. The Journal of Nutrition, Health & Aging, 8(3), 156–162.
Curcio, C. A., Millican, C. L., Allen, K. A., & Kalina, R. E. (1993). Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investigative Ophthalmology & Visual Science, 34(12), 3278–3296.
Damoiseaux, J. S., Beckmann, C. F., Arigita, E. S., Barkhof, F., Scheltens, P., Stam, C. J., ... Rombouts, S. A. R. B. (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex, 18(8), 1856–1864.
Daubechies, I., Roussos, E., Takerkart, S., Benharrosh, M., Golden, C., D'Ardenne, K., Richter, W., Cohen, J. D., & Haxby, J. (2009). Independent component analysis for brain fMRI does not select for independence. Proceedings of the National Academy of Sciences, 106(26), 10415–10422.
Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., & Cabeza, R. (2008). Que PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209.
Dennis, N. A., Daselaar, S., & Cabeza, R. (2007). Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging, 28(11), 1749–1758.
Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M., Bertram, L., Mullin, K., Tanzi, R. E., Blacker, D., Albert, M. S., & Sperling, R. A. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology, 65(3), 404–411. https://doi.org/10.1212/01.wnl.0000171450.97464.49.
Düzel, E., Schütze, H., Yonelinas, A. P., & Heinze, H.-J. (2011). Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus, 21(8), 803–814. https://doi.org/10.1002/hipo.20834.
Elman, J. A., Madison, C. M., Baker, S. L., Vogel, J. W., Marks, S. M., Crowley, S., ... Jagust, W. J. (2016). Effects of beta-amyloid on resting state functional connectivity within and between networks reflect known patterns of regional vulnerability. Cerebral Cortex, 26(2), 695–707.
Erdman, J. W., Smith, J. W., Kuchan, M. J., Mohn, E. S., Johnson, E. J., Rubakhin, S. S., … Neuringer, M. (2015). Lutein and brain function. Foods, 4(4), 547–564. https://doi.org/10.3390/foods4040547.
Feeney, J., Finucane, C., Savva, G. M., Cronin, H., Beatty, S., Nolan, J. M., & Kenny, R. A. (2013). Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiology of Aging, 34(11), 2449–2456. https://doi.org/10.1016/j.neurobiolaging.2013.05.007.
Ferreira, L. K., & Busatto, G. F. (2013). Resting-state functional connectivity in normal brain aging. Neuroscience & Biobehavioral Reviews, 37(3), 384–400. https://doi.org/10.1016/j.neubiorev.2013.01.017.
Franzmeier, N., Caballero, M. A., Taylor, A. N. W., Simon-Vermot, L., Buerger, K., Ertl-Wagner, B., ... Gesierich, B. (2016). Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging and Behavior. 11, 368, 382 https://doi.org/10.1007/s11682-016-9599-1.
Gao, H., & Hollyfield, J. G. (1992). Aging of the human retina: Differential loss of neurons and retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science, 33(1), 1–17.
Garcés, P., Ángel Pineda-Pardo, J., Canuet, L., Aurtenetxe, S., López, M. E., Marcos, A., … Maestú, F. (2014). The default mode network is functionally and structurally disrupted in amnestic mild cognitive impairment — A bimodal MEG–DTI study. NeuroImage, 6, 214–221. https://doi.org/10.1016/j.nicl.2014.09.004.
Ge, B., Makkie, M., Wang, J., Zhao, S., Jiang, X., Li, X., Lv, J., Zhang, S., Zhang, W., Han, J., Guo, L., & Liu, T. (2016). Signal sampling for efficient sparse representation of resting state FMRI data. Brain Imaging and Behavior, 10(4), 1206–1222.
Geerligs, L., Maurits, N. M., Renken, R. J., & Lorist, M. M. (2014). Reduced specificity of functional connectivity in the aging brain during task performance. Human Brain Mapping, 35(1), 319–330. https://doi.org/10.1002/hbm.22175.
Geerligs, L., Renken, R. J., Saliasi, E., Maurits, N. M., & Lorist, M. M. (2015). A brain-wide study of age-related changes in functional connectivity. Cerebral Cortex, 25(7), 1987–1999. https://doi.org/10.1093/cercor/bhu012.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258.
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642. https://doi.org/10.1073/pnas.0308627101.
Hammond, B. R., Johnson, E. J., Russell, R. M., Krinsky, N. I., Yeum, K. J., Edwards, R. B., & Snodderly, D. M. (1997). Dietary modification of human macular pigment density. Investigative Ophthalmology & Visual Science, 38(9), 1795–1801.
Hammond, B. R., Wooten, B. R., & Smollon, B. (2005). Assessment of the validity of in vivo methods of measuring human macular pigment optical density. Optometry and Vision Science, 82(5), 387–404.
Hampson, M., Peterson, B. S., Skudlarski, P., Gatenby, J. C., & Gore, J. C. (2002). Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping, 15(4), 247–262.
Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., … Kummer, M. P. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet: Neurology, 14(4), 388–405. https://doi.org/10.1016/S1474-4422(15)70016-5.
Hibino, H. (1992). Red-green and yellow-blue opponent-color responses as a function of retinal eccentricity. Vision Research, 32(10), 1955–1964.
Jackson, G. R., Owsley, C., Cordle, E. P., & Finley, C. D. (1998). Aging and scotopic sensitivity. Vision Research, 38(22), 3655–3662.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). FSL. Neuroimage, 62(2), 782–790.
Jiang, X., Li, X., Lv, J., Zhang, T., Zhang, S., Guo, L., & Liu, T. (2015). Sparse representation of HCP grayordinate data reveals novel functional architecture of cerebral cortex. Human Brain Mapping, 36(12), 5301–5319.
Johnson, E. J. (2012). A possible role for lutein and zeaxanthin in cognitive function in the elderly. The American Journal of Clinical Nutrition, 96(5), 1161–1165.
Johnson, E. J. (2014). Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutrition Reviews, 72(9), 605–612. https://doi.org/10.1111/nure.12133.
Johnson, E. J., McDonald, K., Caldarella, S. M., Chung, H., Troen, A. M., & Snodderly, D. M. (2008). Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutritional Neuroscience, 11(2), 75–83.
Johnson, E. J., Vishwanathan, R., Johnson, M. A., Hausman, D. B., Davey, A., Scott, T. M., Green, R. C., Miller, L. S., Gearing, M., Woodard, J., Nelson, P. T., Chung, H. Y., Schalch, W., Wittwer, J., & Poon, L. W. (2013). Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia centenarian study. Journal of Aging Research., 2013, 1–13. https://doi.org/10.1155/2013/951786.
Kalkstein, J., Checksfield, K., Bollinger, J., & Gazzaley, A. (2011). Diminished top-down control underlies a visual imagery deficit in normal aging. Journal of Neuroscience, 31(44), 15768–15774.
Khachik, F., Beecher, G. R., Goli, M. B., & Lusby, W. R. (1991). Separation, identification, and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography. Pure and Applied Chemistry, 63(1), 71–80. https://doi.org/10.1351/pac199163010071.
Krinsky, N. I., Mayne, S. T., & Sies, H. (2004). Carotenoids in health and disease. Boca Raton: CRC Press.
Lee, K., Tak, S., & Ye, J. C. (2011). A data-driven sparse GLM for fMRI analysis using sparse dictionary learning with MDL criterion. IEEE Transactions on Medical Imaging, 30(5), 1076–1089.
Lee, Y. B., Lee, J., Tak, S., Lee, K., Na, D. L., Seo, S. W., ... Alzheimer's Disease Neuroimaging Initiative. (2016). Sparse SPM: Group sparse-dictionary learning in SPM framework for resting-state functional connectivity MRI analysis. NeuroImage, 125, 1032–1045.
Li, S. C., Lindenberger, U., & Sikström, S. (2001). Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486.
Li, S. C., Lindenberger, U., Hommel, B., Aschersleben, G., Prinz, W., & Baltes, P. B. (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science, 15(3), 155–163.
Lindbergh, C. A., Mewborn, C., Hammond, B. R., Renzi-Hammond, L. M., Curran-Celentano, J. M., & Miller, L. S. (2016). The relationship of lutein and zeaxanthin levels to neurocognitive functioning: An fMRI study of older adults. Journal of the International Neuropsychological Society, 22, 1–12. https://doi.org/10.1017/S1355617716000850.
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869–878.
Lv, J., Jiang, X., Li, X., Zhu, D., Chen, H., Zhang, T., Zhang, S., Hu, X., Han, J., Huang, H., Zhang, J., Guo, L., & Liu, T. (2015a). Sparse representation of whole-brain fMRI signals for identification of functional networks. Medical Image Analysis, 20(1), 112–134.
Lv, J., Jiang, X., Li, X., Zhu, D., Zhang, S., Zhao, S., Chen, H., Zhang, T., Hu, X., Han, J., Ye, J., Guo, L., & Liu, T. (2015b). Holistic atlases of functional networks and interactions reveal reciprocal organizational architecture of cortical function. IEEE Transactions on Biomedical Engineering, 62(4), 1120–1131.
Lv, J., Jiang, X., Li, X., Zhu, D., Zhao, S., Zhang, T., Hu, X., Han, J., Guo, L., Li, Z., Coles, C., Hu, X., & Liu, T. (2015c). Assessing effects of prenatal alcohol exposure using group-wise sparse representation of fMRI data. Psychiatry Research: Neuroimaging, 233(2), 254–268.
Lv, J., Iraji, A., Ge, F., Zhao, S., Hu, X., Zhang, T., … Liu, T. (2016). Temporal concatenated sparse coding of resting state fMRI data reveal network interaction changes in mTBI. Proceedings of the Medical Image Computing and Computer Assisted Intervention Society, 9900, 46–54.
Ma, L., Dou, H.-L., Wu, Y.-Q., Huang, Y.-M., Huang, Y.-B., Xu, X.-R., … Lin, X.-M. (2012). Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. British Journal of Nutrition, 107(3), 350–359. https://doi.org/10.1017/S0007114511004260.
Mairal, J., Bach, F., Ponce, J., & Sapiro, G. (2010). Online learning for matrix factorization and sparse coding. The Journal of Machine Learning Research, 11, 19–60.
Makkie, M., Zhao, S., Jiang, X., Lv, J., Zhao, Y., Ge, B., Li, X., Han, J., & Liu, T. (2015). HAFNI-enabled largescale platform for neuroimaging informatics (HELPNI). Brain Informatics, 2(4), 225–238.
Malinow, M. R., Feeney-Burns, L., Peterson, L. H., Klein, M. L., & Neuringer, M. (1980). Diet-related macular anomalies in monkeys. Investigative Ophthalmology and Visual Science, 19(8), 857–863.
Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houdé, O., Crivello, F., Joliot, M., Petit, L., & Tzourio-Mazoyer, N. (2001). Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin, 54(3), 287–298.
Owsley, C. (2011). Aging and vision. Vision Research, 51(13), 1610–1622.
Park, D. C., & Reuter-Lorenz, P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. https://doi.org/10.1146/annurev.psych.59.103006.093656.
Park, D. C., Polk, T. A., Park, R., Minear, M., Savage, A., & Smith, M. R. (2004). Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(35), 13091–13095.
Park, D. C., Polk, T. A., Hebrank, A. C., & Jenkins, L. (2010). Age differences in default mode activity on easy and difficult spatial judgment tasks. Frontiers in Human Neuroscience, 3, 75.
Persson, J., Pudas, S., Nilsson, L.-G., & Nyberg, L. (2014). Longitudinal assessment of default-mode brain function in aging. Neurobiology of Aging, 35(9), 2107–2117. https://doi.org/10.1016/j.neurobiolaging.2014.03.012.
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., Vogel, A. C., Laumann, T. O., Miezin, F. M., Schlagger, B. L., & Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678.
Presley, T. D., Morgan, A. R., Bechtold, E., Clodfelter, W., Dove, R. W., Jennings, J. M., Kraft, R. A., Bruce King, S., Laurienti, P. J., Jack Rejeski, W., Burdette, J. H., Kim-Shapiro, D. B., & Miller, G. D. (2011). Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxid: Biology and Chemistry, 24(1), 34–42. https://doi.org/10.1016/j.niox.2010.10.002.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682.
Reiman, E. M., Chen, K., Alexander, G. E., Caselli, R. J., Bandy, D., Osborne, D., Saunders, A. M., & Hardy, J. (2004). Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences, 101(1), 284–289. https://doi.org/10.1073/pnas.2635903100.
Renzi, L. M., & Hammond, B. R. (2010). The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic and Physiological Optics, 30(4), 351–357. https://doi.org/10.1111/j.1475-1313.2010.00720.x.
Renzi, L. M., Iannaccone, A., Johnson, E., & Kritchevsky, S. (2008). The relation between serum xanthophylls, fatty acids, macular pigment and cognitive function in the health ABC study. FASEB Journal, 22, 877.5. https://doi.org/10.1096/fj.1530-6860.
Renzi, L. M., Dengler, M. J., Puente, A., Miller, L. S., & Hammond, B. R. (2014). Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiology of Aging, 35(7), 1695–1699. https://doi.org/10.1016/j.neurobiolaging.2013.12.024.
Reuter-Lorenz, P. A., & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182.
Reuter-Lorenz, P. A., & Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 24(3), 355–370. https://doi.org/10.1007/s11065-014-9270-9.
Rosano, C., Marsland, A. L., & Gianaros, P. J. (2012). Maintaining brain health by monitoring inflammatory processes: A mechanism to promote successful aging. Aging and Disease, 3(1), 16–33.
Sala-Llonch, R., Bartrés-Faz, D., & Junqué, C. (2015). Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology, 6, 1–11. https://doi.org/10.3389/fpsyg.2015.00663.
Sambataro, F., Murty, V. P., Callicott, J. H., Tan, H. Y., Das, S., Weinberger, D. R., & Mattay, V. S. (2010). Age-related alterations in default mode network: Impact on working memory performance. Neurobiology of Aging, 31(5), 839–852.
SanGiovanni, J. P., & Neuringer, M. (2012). The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: Promise of molecular genetics for guiding mechanistic and translational research in the field. The American Journal of Clinical Nutrition, 96(5), 1223–1233. https://doi.org/10.3945/ajcn.112.038240.
Sartorius, T., Ketterer, C., Kullmann, S., Balzer, M., Rotermund, C., Binder, S., Hallschmid, M., Machann, J., Schick, F., Somoza, V., Preissl, H., Fritsche, A., Haring, H. U., & Hennige, A. M. (2012). Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes, 61(7), 1669–1679. https://doi.org/10.2337/db11-1521.
Schefrin, B. E., Hauser, M., & Werner, J. S. (2004). Evidence against age-related enlargements of ganglion cell receptive field centers under scotopic conditions. Vision Research, 44(4), 423–428.
Schmidt, A., Hammann, F., Wölnerhanssen, B., Meyer-Gerspach, A. C., Drewe, J., Beglinger, C., & Borgwardt, S. (2014). Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology, 231(19), 3879–3888. https://doi.org/10.1007/s00213-014-3526-1.
Sheline, Y. I., & Raichle, M. E. (2013). Resting state functional connectivity in preclinical Alzheimer’s disease. Biological Psychiatry, 74(5), 340–347.
Sheline, Y. I., Raichle, M. E., Snyder, A. Z., Morris, J. C., Head, D., Wang, S., & Mintun, M. A. (2010). Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry, 67(6), 584–587. https://doi.org/10.1016/j.biopsych.2009.08.024.
Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E., & Petersen, S. E. (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience, 9(5), 648–663.
Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., Filippini, N., Watkins, K. E., Toro, R., Laird, A. R., & Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045.
Snodderly, D. M., Auran, J. D., & Delori, F. C. (1984a). The macular pigment. II. Spatial distribution in primate retinas. Investigative Ophthalmology & Visual Science, 25(6), 674–685.
Snodderly, D. M., Brown, P. K., Delori, F. C., & Auran, J. D. (1984b). The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investigative Ophthalmology & Visual Science, 25(6), 660–673.
Spaniol, J., & Grady, C. (2012). Aging and the neural correlates of source memory: Over-recruitment and functional reorganization. Neurobiology of Aging, 33(2), 3–18.
Spreng, R. N., & Schacter, D. L. (2012). Default network modulation and large-scale network interactivity in healthy young and old adults. Cerebral Cortex, 22(11), 2610–2621.
Stahl, W., & Sies, H. (2001). Effects of carotenoids and retinoids on gap junctional communication. Biofactors, 15(2–4), 95–98.
Stringham, J. M., & Hammond, B. R. (2007). Compensation for light loss due to filtering by macular pigment: Relation to hue cancellation. Ophthalmic and Physiological Optics, 27(3), 232–237.
Stringham, J. M., Hammond, B. R., Nolan, J. M., Wooten, B. R., Mammen, A., Smollon, W., & Snodderly, D. M. (2008). The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Experimental Eye Research, 87(5), 445–453.
Tyler, L. K., Shafto, M. A., Randall, B., Wright, P., Marslen-Wilson, W. D., & Stamatakis, E. A. (2010). Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex, 20(2), 352–364. https://doi.org/10.1093/cercor/bhp105.
Vishwanathan, R., Neuringer, M., Snodderly, D. M., Schalch, W., & Johnson, E. J. (2013). Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutritional Neuroscience, 16(1), 21–29. https://doi.org/10.1179/1476830512Y.0000000024.
Vishwanathan, R., Iannaccone, A., Scott, T. M., Kritchevsky, S. B., Jennings, B. J., Carboni, G., Forma, G., Satterfield, S., Harris, T., Johnson, K. C., Schalch, W., Renzi, L. M., Rosano, C., & Johnson, E. J. (2014). Macular pigment optical density is related to cognitive function in older people. Age and Ageing, 43(2), 271–275. https://doi.org/10.1093/ageing/aft210.
Vishwanathan, R., Schalch, W., & Johnson, E. J. (2016). Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutritional Neuroscience, 19(3), 95–101. https://doi.org/10.1179/1476830514Y.0000000141.
Voss, M. W., Prakash, R. S., Erickson, K. I., Basak, C., Chaddock, L., Kim, J. S., … Kramer, A. F. (2010). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience, 2, 1–17. https://doi.org/10.3389/fnagi.2010.00032.
Wang, L., LaViolette, P., O’Keefe, K., Putcha, D., Bakkour, A., Van Dijk, K. R. A., … Sperling, R. A. (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage, 51(2), 910–917. https://doi.org/10.1016/j.neuroimage.2010.02.046.
Wechsler, D. (2001). Wechsler Test of Adult Reading: WTAR. San Antonio: Psychological Corporation.
Widomska, J., & Subczynski, W. K. (2014). Why has nature chosen lutein and zeaxanthin to protect the retina? Journal of Clinical & Experimental Ophthalmology, 5(1). https://doi.org/10.4172/2155-9570.1000326.
Xiong, J., Parsons, L. M., Gao, J. H., & Fox, P. T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping, 8(2–3), 151–156.
Yehuda, S., Rabinovitz, S., Carasso, R., & Mostofsky, D. (2002). The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiology of Aging, 23(5), 843–853. https://doi.org/10.1016/S0197-4580(02)00074-X.
Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., & Leirer, V. O. (1983). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. https://doi.org/10.1016/0022-3956(82)90033-4.
Zamroziewicz, M. K., & Barbey, A. K. (2016). Nutritional cognitive neuroscience: Innovations for healthy brain aging. Frontiers in Neuroscience, 10, 1–10. https://doi.org/10.3389/fnins.2016.00240.
Zhao, Y., Chen, H., Li, Y., Lv, J., Jiang, X., Ge, F., Zhang, T., Zhang, S., Ge, B., Lyu, C., Zhao, S., Han, J., Guo, L., & Liu, T. (2016). Connectome-scale group-wise consistent resting-state network analysis in autism spectrum disorder. NeuroImage: Clinical, 12, 23–33.
Funding
This research project was funded in part by Abbott Nutritional Products (Columbus, OH; research grant to B.R.H., L.M.R., L.S.M.) and the University of Georgia’s Bio-Imaging Research Center (Athens, GA; administrative support to L.S.M.). DSM Nutritional Products (Switzerland) provided the supplements and placebos.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.M.R. was an employee of Abbott Nutrition during a portion of the grant period while holding a joint appointment at the University of Georgia. B.R.H. has consulted for Abbott Nutrition. No other potential conflicts of interest exist for any of the study authors, including A.N.P., C.A.L., C.M.M., D.P.T, J.L., T.L., and Y.Z. The study design, the collection, analysis, and interpretation of the data, and the writing of the report were all completed independently of supporting agencies.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 169 kb)
Rights and permissions
About this article
Cite this article
Lindbergh, C.A., Lv, J., Zhao, Y. et al. The effects of lutein and zeaxanthin on resting state functional connectivity in older Caucasian adults: a randomized controlled trial. Brain Imaging and Behavior 14, 668–681 (2020). https://doi.org/10.1007/s11682-018-00034-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-00034-y