Abstract
In this work, the effect of boron content on the high-temperature wetting behavior in the Si-B alloy/h-BN systems was experimentally examined. For this reason, hypoeutectic, eutectic and hypereutectic Si-B alloys (Si-1B, Si-3.2B and Si-5.7B wt.%, respectively) were produced by electric arc melting method and then subjected to sessile drop/contact heating experiments with polycrystalline h-BN substrates, at temperatures up to 1750 °C. Similar to pure Si/h-BN system, wetting kinetics curves calculated on a basis of in situ recorded drop/substrate images point toward non-wetting behavior of all selected Si-B alloy/h-BN couples. The highest contact angle values of ~ 150° were obtained for hypoeutectic and eutectic Si-B alloys in the whole examined temperature range.
Similar content being viewed by others
Introduction
Due to the high melting points and latent heat values of silicon and boron, Si-B alloys have been assumed as excellent candidates to be used as phase change materials (PCMs) for applications in latent heat thermal energy storage (LHTES) and conversion systems working at the temperatures up to 2000 °C (Ref 1). The main idea of a LHTES-based system is that the latent heat, absorbed during melting and released upon solidification, can be stored and converted into other forms of energy, e.g., electricity. The basic idea of AMADEUS project (Ref 1), supported by the European Commission within Horizon 2020 Programme, is that the utilization of latent heat of Si-B alloys should significantly overcome energy density limitations of the present molten salt-based LHTES systems (Fig. 1). However, in order to successfully accomplish the project goals, some scientific and technological challenges need to be a priori faced and solved. Besides the development of new ultra-high-temperature devices for a heat-electricity conversion [i.e., thermoionic photovoltaic converter (Ref 1)], proper ceramic materials able to withstand a long-term heating, holding and cooling in contact with molten Si-B alloys need to be selected. Thus, an implementation of these materials to real devices must be preceded by careful examinations of their high-temperature interaction with refractories that can be used for building the PCM container.
A possible energy density coming from latent heat of different materials as a function of melting temperature compared with the energy density of other storage technologies (based on Ref 1)
Firstly, to achieve long lifetimes and high reliability of the PCM container, it must be inert toward contacting Si-B alloys under operating conditions. In other words, a selection of proper refractories should be based on two main criteria: (1) a non-wettability and (2) a negligible reactivity in contact with Si-B melts. However, due to the pioneering nature of research, there is a lack of both theoretical and experimental data on interaction between molten Si-B alloys and ceramics at any temperature. On the other hand, high-temperature capillarity phenomena (i.e., wetting, spreading and infiltration) have been so far widely examined for Si/ceramic systems in terms of a fabrication of photovoltaic grade silicon (Ref 2, 3). It has been documented that at a temperature near melting point of Si (Tm = 1414 °C), almost all applied in practice ceramics, e.g., oxides, carbides, borides or nitrides, are well wetted by molten Si, as reflected by the contact angle θ < 90°. This behavior originates from a high chemical affinity of Si to oxygen, carbon and nitrogen leading to an easy formation of reaction products at the interfaces of contacting materials. In fact, the only one available ceramic showing the non-wetting behavior with molten Si is hexagonal boron nitride (h-BN). The reported values of the equilibrium contact angle θ measured for Si/h-BN couples at T < 1500 °C are within the range of 95°-145° (e.g., Ref 4, 5). In our previous work (Ref 6), it was found that the non-wetting behavior of the Si/h-BN system is maintained up to 1650 °C, while a non-wetting-to-wetting transition takes place at higher temperatures. It has also been evidenced that the involved reactivity mechanism under static argon atmosphere is mostly based on the h-BN dissolution in molten Si (the dissolution rate depends on temperature), followed by a reprecipitation of h-BN platelets during cooling. Additionally, the interaction was accompanied by the formation of gaseous product (most probably N2 but also B2O3 introduced from the batch powders or during sintering step), which was reflected by a lack of completely equilibrated conditions.
In this work, we used the sessile drop method experiments to examine the effect of boron content on the high-temperature interaction (wetting and spreading) during contact heating of Si-B alloys with hexagonal boron nitride (h-BN) substrates at temperatures up to 1750 °C.
Materials and Methods
The nominal compositions of Si-B alloys (Fig. 2a) were selected in accordance with the Si-B binary phase diagram proposed by Olesinski and Abbaschian (Ref 7) (Fig. 2b) as hypoeutectic (Si-1B), eutectic (Si-3.2B) and hypereutectic (Si-5.7B) (wt.%). Additionally, ultra-highly pure Si (7N) was used as the reference material (Ref 6).
A macroview of exemplary Si-B alloys fabricated by using the electric arc melting method (a). The Si-B binary phase diagram proposed by Olesinski and Abbaschian (Ref 7) with marked composition of produced alloys
The Si-B alloy samples with a mass of ~ 0.6 g were produced from polycrystalline pure materials (Si: 99.999%; B: 99.9%—provided by Onyxmet, Poland). The main impurities in the batch materials were Al (0.008 at.%) and P (0.002 at.%) in silicon and Fe (0.023 at.%) and Al (0.012 at.%) in boron. The alloys were fabricated by the electric arc melting technique (Buehler Arc Melter MAM-1) by using properly weight mixtures of pure elements. The prepared Si-B mixtures were heated up in argon protective atmosphere until a complete melting and then rapidly cooled down after switching off the electric arc. In order to increase homogeneity of the Si-B alloys, each sample was twice remelted in the arc melting device.
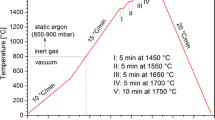
Commercially available h-BN sinters (HeBoSint D100, Henze Boron Nitride Products AG, Lauben, Germany) were used as the substrates in the wetting tests. Before experiments, contacting surfaces of h-BN substrates were gently ground and polished to achieve the surface roughness of ~ 150 nm. The sessile drop experiments were performed with a dedicated experimental complex (Ref 8) working under argon atmosphere, in the temperature range between 1450 and 1750 °C (the applied temperature profile is shown in Fig. 3). In these tests, in order to mimic real working conditions of a PCM device, static argon atmosphere (p = 850-900 mbar) was applied.
Temperature profile (heating/holding/cooling scheme) of the sessile drop experiment (more details are shown in Ref 6)
More details on experimental procedure are given elsewhere (Ref 6). During the tests, the images of Si-B/h-BN couples were recorded at 100 fps by using a high-speed high-resolution CDD camera. Subsequently, the acquired images were post-processed to compile movies and calculate wetting kinetics. After the tests, the solidified couples were removed from the vacuum chamber and subjected to structural evaluations. The characterization of Si-B alloy in as-fabricated state and Si-B/h-BN sessile drop couples was carried out by using Carl Zeiss Axio Observer ZM10 light microscope and FEI Scios™ field emission gun scanning electron microscope (FEGSEM) coupled with energy-dispersive x-ray spectroscopy (EDS).
Results and Discussion
Characterization of Si-B Alloys in As-Fabricated State
The results of microstructural characterization of the as-fabricated Si-B alloys are shown in Fig. 4. SEM images of the cross-sectioned alloys revealed that despite using double remelting, the microstructure of alloys is quite inhomogeneous, while a degree of inhomogeneity increased with increasing boron content. A matrix of each alloy was composed of the mixture of Si(B) solid solution and Si + SiB3 eutectic. With increasing boron content, the number of boron-rich precipitates also increases. The results of SEM/EDS local chemical composition analyses revealed a B/Si atomic ratio of 3.01 ± 0.15 in the eutectic areas and B/Si = 4.11 ± 0.13 in dark gray particles. Additionally, black particles were found to be almost Si-free and they exhibited B/C ratio of 4.07 ± 0.09. Therefore, the following structural features were recognized based on careful SEM/EDS examinations: Si(B) solid solution, Si + SiB3 eutectic, SiB4 silicon tetraboride and B4C boron carbide.
It should be noted that serious discrepancies on boron-rich part of the Si-B phase diagram are reported in the literature. The most contradictory findings have been shown for the phase stability and stoichiometry of silicon borides. Although the widely accepted form of Si-B phase diagram (Ref 7) contains only three borides, namely SiB3, SiB6 and SiBn, some earlier and more recent papers, e.g., by Samsonov and Sleptsov (Ref 9) or by Tremblay and Angers (Ref 10, 11), point toward the existence of SiB4 instead of SiB3 compound, while other researchers, e.g., Aselage (Ref 12), reported that the SiB3 phase grows from boron saturated silicon, but at the same time they indicated its metastability toward SiB6 phase. On the other hand, it has been proposed (Ref 13) that the triboride is a silicon-rich version of the tetraboride, so the stoichiometry of either compound could be expressed as SiB4-x where x = 0 or 1. Additionally, due to local segregations of chemical composition, both phases (tri- and tetraboride) might also coexist.
Thus, it seems that presently applied non-equilibrium solidification conditions of electric arc melting processing (i.e., rapid quenching and high solidification rates) supported the formation of SiB4 as the most thermodynamically stable phase, while the presence of B4C particles should be justified in terms of carbon impurities introduced from the batch materials.
The Wetting Kinetics in Si-B Alloy/h-BN Systems
The images of Si/h-BN and Si-B/h-BN sessile drop couples in situ recorded during the high-temperature tests are shown in Fig. 5. The wetting kinetics curves (showing a change of contact angle θ vs. testing time) calculated for pure silicon and Si-B alloys subjected to contact heating with h-BN substrates are shown in Fig. 6. By comparing the results obtained for Si-B alloys to the behavior of pure silicon on the h-BN substrate (described in details in Ref 6), it is concluded that the additional presence of boron decreases the wettability in the system. For all Si-B alloys, the contact angle values were very high (within the non-wetting regime of θ > 90°) in the whole examined temperature range.
Wetting kinetics curves (showing a change of contact angle θ vs. testing time) calculated for Si-B alloys subjected to contact heating with h-BN substrates (data for pure Si are taken from Ref 6)
However, it should be noted that the wetting kinetics curve for the Si-5.7B hypereutectic alloy showed a noticeable decrease from θ = 145° at 1450 °C to θ = 125° at 1750 °C. Most probably, this behavior might be attributed to an inhomogeneous initial structure of the Si-5.7B alloy, in particular to the presence of relatively large SiB4 (and B4C) crystals distributed in the bottom part of the alloy (Fig. 4c) directly contacting the h-BN substrate. Due to a high chemical affinity of Si(B) melt to both SiB4 and B4C phases reflected also by a very good wetting, it is believed that the existence of this “discontinuous layer” in the vicinity of Si-5.7B/h-BN interface might be responsible for the observed decrease in contact angle θ. This finding allows concluding that increased fraction of high melting point borides in Si-B alloys having the hypereutectic composition is not beneficial in terms of the “non-wettability requirement” for the selection of container materials in LHTES device. Furthermore, what is extremely important from the application’s point of view, a large amount of crystals having high melting points decreases a relative content of liquid phase providing the latent heat for the electricity generation.
As it has been experimentally shown in earlier work (Ref 6) during the high-temperature interaction in Si/h-BN system, h-BN substrate is slightly dissolved in initially pure Si, leading to diffusion of boron into molten Si. In view of this finding, it is reasonable to conclude that addition of boron to silicon before the experiment (i.e., using Si-B alloys instead of pure Si) suppresses this phenomenon. In other words, the Si-B alloys dissolve much less boron from the h-BN substrate, which results in a remarkable hindering of substrate dissolution and a fast achievement of a thermodynamic equilibrium.
This statement seems to be also confirmed by a strikingly different behavior of Si/h-BN and Si-B /h-BN couples during cooling from 1750 °C. In the former case, an increase in contact angle θ upon cooling (a so-called dewetting) was observed as the effect of drastic change in solubility of previously diffused B, N and C atoms in liquid/solid-state silicon. Consequently, a release of gaseous nitrogen and precipitation of BN platelets and SiC crystals take place at the Si/h-BN interface during the solidification. On the other hand, Si-B alloys exhibited either the negligible alteration of the θ versus t curve (for Si-1B alloy) or its descending tendency (for Si-3.2B and Si-5.7B alloys) during cooling. Decreasing the contact angle θ during cooling of eutectic and hypereutectic Si-B alloys should be justified by the change in structure and chemistry of the interface due to the formation of wettable silicon boride crystals at the interface area before the solidification of Si(B) matrix, i.e., Si-B/h-BN system was locally converted to Si-B/B4C+SiBx /h-BN. The results of LM and SEM/EDS analyses of cross-sectioned solidified couples (Fig. 7) revealed that:
-
(1)
the size and number of silicon boride crystals in the interface vicinity increase with increasing initial boron content in Si-B alloys (Fig. 7a-c);
-
(2)
the EDS estimated chemical composition of the large gray crystals is very close to the stoichiometry of SiB3 triboride. Furthermore, in the case of the Si-5.7B hypereutectic alloy (Fig. 7d), the presence of few dark crystals having the B/Si ratio of ~ 6.18 ± 0.15 (corresponding to the SiB6 hexaboride) was also noted. This finding suggests that under conditions of cooling rates slower than that in the electric arc melting process, the SiB3 and SiB6 phases may coexist as more stable than the silicon tetraboride.
In addition, boron has been already recognized as the surface active element in many metal-boron systems (Ref 14), which means that B atoms preferentially segregate at the liquid–vapor interface (Ref 15). Therefore, the observed decrease in contact angle during cooling of Si-B alloys might be also related to a probably negative effect of increased boron content on the melt surface tension.
Conclusions
The following conclusions are drawn from the obtained experimental results and supported by appropriate data from the literature:
-
1.
Silicon–boron alloys having various boron contents were successfully fabricated by electric arc melting process. Based on the results of SEM/EDS analyses, following structural features were recognized in the as-fabricated alloys: Si(B) solid solution, Si + SiB3 eutectic, SiB4 silicon tetraboride and B4C boron carbide.
-
2.
The wettability tests for Si-B alloys/h-BN couples were performed for the first time by sessile drop experiments at temperatures up to 1750 °C. It was established that the Si-B alloys exhibited much lower wettability with the h-BN ceramic as compared to the pure silicon counterpart. For both Si-1B hypoeutectic and Si-3.2B eutectic alloys, very high contact angle θ values of ~ 150° were recorded in the whole examined temperature range. The Si-5.7B hypereutectic alloy shows slightly lower contact angle θ values, most probably due to its inhomogeneous initial structure including the presence of primary high melting point borides in the vicinity of the surface contacting the h-BN substrate.
-
3.
Since wetting phenomenon in Si/h-BN system at ultra-high temperature is dominated by the dissolution of h-BN in molten Si followed by reprecipitation during cooling, the lack of wetting in Si-B alloy/h-BN system under the same conditions as for pure Si/h-BN is caused by a suppression of this mechanism.
-
4.
Regarding the predicted application of Si-B/h-BN systems in the ultra-high-temperature LHTES devices, it is suggested to use hypoeutectic or near eutectic compositions of Si-B alloys. This choice is justified not only by presently documented negligible interaction with the h-BN ceramic, but also by theoretically highest available latent heat for these alloys (Ref 1).
References
A. Datas, A. Ramos, A. Marti, C. del Canizo, and A. Luque, Ultra High Temperature Latent Heat Energy Storage and Thermophotovoltaic Energy Conversion, Energy, 2016, 107, p 542–549
B. Drevet and N. Eustathopoulos, Wetting of Ceramics by Molten Silicon and Silicon Alloys: A Review, J. Mater. Sci., 2012, 47, p 8247–8260
Z. Yuan, W.I. Huang, and K. Mukai, Wettability and Reactivity of Molten Silicon with Various Substrates, Appl. Phys. A Mater., 2004, 78, p 617–622
J.A. Champion, B.J. Keene, and S. Allen, Wetting of Refractory Materials by Molten Metallides, J. Mater. Sci., 1973, 8, p 423–426
B. Drevet, R. Voytovych, R. Israel, and N. Eustathopoulos, Wetting and Adhesion of Si on Si3N4 and BN Substrates, J. Eur. Ceram. Soc., 2009, 29, p 2363–2367
W. Polkowski, N. Sobczak, R. Nowak, A. Kudyba, G. Bruzda, A. Polkowska, M. Homa, P. Turalska, M. Tangstad, J. Safarian, E. Moosavi-Khoonsari, and A. Datas, Wetting Behavior and Reactivity of Molten Silicon with h-BN Substrate at Ultrahigh Temperatures up to 1750 °C, J. Mater. Eng. Perform., 2018, 27, p 5040–5053
R.W. Olesinski and G.J. Abbaschian, The B-Si (Boron-Silicon) System, Bull. Alloys Phase Diagr., 1984, 5, p 479–484
N. Sobczak, R. Nowak, W. Radziwill, J. Budzioch, and A. Glenz, Experimental Complex for Investigations of High Temperature Capillarity Phenomena, Mater. Sci. Eng. A, 2008, 495, p 43–49
G.V. Samsonov and V.M. Sleptsov, Preparation of Boron-Silicon Alloys, Powder Metall. Met. Ceram., 1964, 3, p 488–496
R. Tremblay and R. Angers, Preparation of High Purity SiB4 by Solid State Reaction Between Si And B, Ceram. Int., 1989, 15, p 73–78
R. Tremblay and R. Angers, Mechanical Characterization of Dense Silicon Tetraboride (SiB4), Ceram. Int., 1992, 18, p 113–117
T.L. Aselage, The Coexistence of Silicon Borides with Boron-Saturated Silicon: Metastability of SiB3, J. Mater. Res., 1998, 13, p 1786–1794
C. Brosset and B. Magnusson, The Silicon-Boron System, Nature, 1960, 187, p 54–55
A.F. Vishkarev, Y.V. Kryakovskii, S.A. Bliznukov, V.I. Yavoiski, Influence of Rare-Earth Elements on the Surface Tension of Liquid Iron, in Surface Phenomena in Metallurgical Processes: Proceedings of an Interinstitute Conference, ed. by A.I. Belyaev (Springer, US, 1965), pp. 166–171
A. Passerone, M.L. Muolo, F. Valenza, and R. Novakovic, Thermodynamics and Surface Properties of Liquid Cu-B Alloys, Surf. Sci., 2009, 603, p 2725–2733
Acknowledgments
The project AMADEUS has received funds from the European Union’s Horizon 2020 research and innovation program, FET-OPEN action, under Grant Agreement 737054. The sole responsibility for the content of this publication lies with the authors. It does not necessarily reflect the opinion of the European Union. Neither the REA nor the European Commission is responsible for any use that may be made of the information contained therein. The authors wish to express their thanks to S. Donath for the help with sample preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the 73rd World Foundry Congress and has been expanded from the original presentation. 73WFC was held in Krakow, Poland, September 23–27, 2018, and was organized by the World Foundry Organization and Polish Foundrymen’s Association.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Polkowski, W., Sobczak, N., Bruzda, G. et al. The Effect of Boron Content on Wetting Kinetics in Si-B Alloy/h-BN System. J. of Materi Eng and Perform 28, 3819–3825 (2019). https://doi.org/10.1007/s11665-018-3786-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3786-8