Abstract
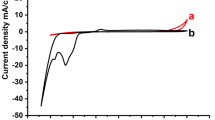
A thick film of cobalt triantimonide (CoSb3) skutterudites was successfully synthesized via electrochemical deposition by using an electrolyte that contains antimony (Sb3+) and cobalt (Co2+) ions in a weak acid solution in the presence of polyvinyl alcohol (PVA) as an additive. The process was conducted under a potentiostatic condition of − 1000 mV versus Ag/AgCl at room temperature. Two hours (2 h) of electrochemical deposition produced a 13 μm assisted PVA CoSb3 thick film. In comparison, most studied electrochemical deposition of CoSb3 skutterudites thin films is in the thickness range of 700–800 nm. The morphological studies of the films appeared to have a cauliflower-like structure with a presence of fern-like dendrite for a non-PVA electrolyte due to the limitation of ion diffusion in the nucleation growth area. The absence of dendrite was observed in the assisted PVA films, which demonstrated an enhanced ion diffusion in the vicinity with a better stoichiometric ratio for Co:Sb of 1:3 thick film.
Similar content being viewed by others
References
Z. Zheng, F. Li, F. Li, Y. Li, P. Fan, J. Luo, G. Liang, B. Fan, and A. Zhong, Thin Solid Films 632, 88 (2017).
M. Rull-Bravo, A. Moure, J.F. Fernández, and M. Martín-González, RSC Adv. 5, 41653 (2015).
T. Caillat, J.-P. Fleurial, and A. Borshchevsky, J. Cryst. Growth 166, 722 (1996).
D.T. Morelli, T. Caillat, J.P. Fleurial, A. Borshchevsky, J. Vandersande, B. Chen, and C. Uher, Phys. Rev. B 51, 9622 (1995).
L. Bertini, K. Biliquist, M. Christensen, C. Gatti, L. Holmgren, B. Iversen, E. Mueller, M. Muhammed, G. Noriega, A. Palmqvist, D. Platzek, D.M. Rowe, A. Saramat, C. Stiewe, M. Toprak, S.G. Williams, and Y. Zhang, in 22nd International Conference on Thermoelectrics proceeding (2003), pp. 93–96.
H. Li, X. Tang, X. Su, and Q. Zhang, Appl. Phys. Lett. 92, 202114 (2008).
G. Rogl, A. Grytsiv, P. Rogl, N. Peranio, E. Bauer, M. Zehetbauer, and O. Eibl, Acta Mater. 63, 30 (2014).
S. Yadav, B.S. Yadav, S. Chaudhary, and D.K. Pandya, RSC Adv. 7, 20336 (2017).
R. Vidu, M. Perez-Page, D.V. Quach, X.Y. Chen, and P. Stroeve, Electroanalysis 27, 2845 (2015).
H. Cheng, H.H. Hng, J. Ma, and X.J. Xu, J. Mater. Res. 23, 3013 (2008).
Y.N. Sadana and R. Kumar, Surf. Technol. 11, 37 (1980).
D.V. Quach, R. Vidu, J.R. Groza, and P. Stroeve, Ind. Eng. Chem. Res. 49, 11385 (2010).
J.F. Behnke, A.L. Prieto, A.M. Stacy, and T. Sands, in Eighteenth International Conference on Thermoelectrics. Proceedings (1999), pp. 451–453.
L.J. Chen, H.N. Hu, Y.X. Li, G.F. Chen, S.Y. Yu, and G.H. Wu, Chem. Lett. 35, 170 (2006).
L. Hicks and D. Dresselhaus, Phys. Rev. B 47, 727 (1993).
C. Lei, K.S. Ryder, E. Koukharenko, M. Burton, and I.S. Nandhakumar, Electrochem. Commun. 66, 1 (2016).
S. Li, H.M.A. Soliman, J. Zhou, M.S. Toprak, M. Muhammed, D. Platzek, P. Ziolkowski, and E. Müller, Chem. Mater. 20, 4403 (2008).
J.H. We, S.J. Kim, G.S. Kim, and B.J. Cho, J. Alloys Compd. 552, 107 (2013).
Y. Ma, W. Wijesekara, and A.E.C. Palmqvist, J. Electron. Mater. 41, 1138 (2012).
N.H. Trung, K. Sakamoto, N.V. Toan, and T. Ono, Materials 10, 154 (2017).
M.A. Hossain, R. Al-Gaashani, H. Hamoudi, M.J. Al Marri, I.A. Hussein, A. Belaidi, B.A. Merzougui, F.H. Alharbi, and N. Tabet, Mater. Sci. Semicond. Process. 63, 203 (2017).
M.H. Tran, J.Y. Cho, S. Sinha, M.G. Gang, and J. Heo, Thin Solid Films 661, 132 (2018).
Q. Zhang, Y. Wang, W. Wang, N. Mitsuzak, and Z. Chen, Electrochem. Commun. 63, 22 (2016).
R. Oommen, U. Rajalakshmi, and Sanjeeviraja, Int. J. Electrochem. Sci. 7, 8288 (2012).
A. Aldalbahi, E.M. Mkawi, K. Ibrahim, and M.A. Farrukh, Sci. Rep. 6, 32431 (2016).
J. Yu, H. Deng, J. Tao, L. Chen, H. Cao, L. Sun, P. Yang, and J. Chu, Mater. Lett. 191, 186 (2017).
H.H. Gatzen, V. Saile, and J. Leuthold, Micro and Nano Fabrication (Berlin: Springer, 2015), p. 65.
Y. Liu, Z. Li, Y. Wang, and W. Wang, Trans. Nonferrous Met. Soc. China 24, 876 (2014).
P.T. Kalisman, Study of the Electrochemical System of Antimony-Tellurium in Dimethyl Sulfoxide for Growth of Nanowire Arrays, and an Innovative Method for Single Nanowire Measurements (Open Access Publications from the University of California, 2012), https://escholarship.org/uc/item/8cr738f5. Accessed 18 Dec 2018.
A. Brenner and G.E. Riddell, J. Res. Natl. Bur. Stand. (U. S.) 39, 385 (1946).
M. Paunovic and M. Schlesinger, Fundamental of Electrochemical Deposition (Hoboken: Wiley, 2006), p. 113.
A. Bweick, M. Fleischmann, and H. Thirsk, Trans. Farad. Soc. 58, 2200 (1962).
M. Paunovic and M. Schlesinger, Fundamental of Electrochemical Deposition (Hoboken: Wiley, 2006), p. 273.
R. Vidu, S. Li, D.V. Quach, and P. Stroeve, J. Appl. Electrochem. 42, 333 (2012).
Y. Sun, J.-L. Sang, X. Wang, and Y.-J. Li, Electrochim. Acta 216, 88 (2016).
E. Kaniukov, D. Yakimchuk, G. Arzumanyan, H. Terryn, K. Baert, A. Kozlovskiy, M. Zdorovets, E. Belonogov, and S. Demyanov, Philos. Mag. 97, 2268 (2017).
M.V. Mandke, S.-H. Han, and H.M. Pathan, CrystEngComm 14, 86 (2012).
D.K. Sharma, A. Ott, A.P. O’Mullane, and S.K. Bhargava, Colloids Surf. A. 386, 98 (2011).
I. Oftedal, Z. Kristallogr. Cryst. Mater. 66, 517 (1928).
M. Paunovic and M. Schlesinger, Fundamental of Electrochemical Deposition (Hoboken: Wiley, 2006), p. 190.
P.B. Patil, S.S. Mali, V.V. Kondalkar, R.M. Mane, P.S. Patil, C.K. Hong, and P.N. Bhosale, J. Electroanal. Chem. 758, 178 (2015).
X. Luo, J. Li, and X. Lin, Carbohydr. Polym. 90, 1595 (2012).
D.I. Tishkevich, S.S. Grabchikov, L.S. Tsybulskaya, V.S. Shendyukov, S.S. Perevoznikov, S.V. Trukhanov, E.L. Trukhanov, A.V. Trukhanov, and D.A. Vinnik, J. Alloys Compd. 735, 1943 (2018).
W. Sang, Y. Fang, J. Fan, Y. He, J. Min, and Y. Qian, J. Cryst. Growth 299, 272 (2007).
C. Lei, M.R. Burton, and I.S. Nandhakumar, Phys. Chem. Chem. Phys. 18, 14164 (2016).
H. Nishikiori, M. Takei, K. Oki, S. Takano, N. Tanaka, and T. Fujii, Appl. Catal. B 127, 227 (2012).
Z. Zheng, P. Fan, G. Liang, and D. Zhang, J. Alloys Compd. 619, 676 (2015).
B. Alinejad, A. Castellero, and M. Baricco, Scr. Mater. 113, 110 (2016).
J. Leszczynski, V.D. Ros, B. Lenoir, A. Dauscher, C. Candolfi, P. Masschelein, J. Hejtmanek, K. Kutorasinski, J. Tobola, R.I. Smith, C. Stiewe, and E. Müller, J. Phys. D Appl. Phys. 46, 495106 (2013).
Acknowledgments
This project was financially supported by University Malaya (PG120-2016A & GPF003A-2018). Part of this work was performed in the Micro/Nanomachining Research Education Center (MNC) of Tohoku University, Japan under a Japan Student Services Organization (JASSO) scholarship. Nuur Syahidah Sabran would like to thank the Ministry of Education of Malaysia for the scholarship (MyPhD) awarded.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabran, N.S., Fadzallah, I., Ono, T. et al. Preparation and Characterization of Electrochemical Deposition Cobalt Triantimonide (CoSb3) Thick Film: Effects of Polyvinyl Alcohol (PVA) as an Additive. J. Electron. Mater. 48, 5003–5011 (2019). https://doi.org/10.1007/s11664-019-07295-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07295-3