Abstract
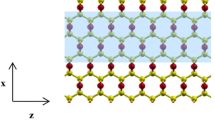
The electronic properties of armchair graphene oxide nanoribbons (AGONRs) with different doped oxygen configurations are studied based on density functional theory using first principle calculations. The electronic properties of the AGONRs are tuned by different oxygen configurations for top edges, center, bottom edges and fifth width. The AGONRs for top-edge O doping configuration are indirect band gap semiconductors with an energy gap of 1.268 eV involving hybridization among C-2p and O-2s, 2p electrons and electrical conductivity of oxygen atoms. The center and bottom edges are direct band gap semiconductors with 1.317 eV and 1.151 eV, respectively. The valence band is contributed from C-2p, O-2p and H-1s for top-edge O doping. The electronic properties of AGONRs are changed due to localization in −2.94 eV of O-2p states. The center O-doped AGONRs are n-type semiconductors with Fermi levels near the conduction band bottom. This is due to hybridization among C-2s, 2p and O-2p electrons. However, bottom-edge O-doped AGONRs are p-type semiconductors, due to the electrical conductivity of oxygen atoms. The fifth-width O-doped AGONRs are indirect band gap semiconductors with an energy gap of 0.375 eV. The projected density of states shows that the localization and hybridization between C-2 s, 2p, O-2p and H-1s electronic states are rising in the conduction band and valence band from the projected density of states. The localization is induced by O-2p electronic states at a Fermi level.
Similar content being viewed by others
References
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, and A.A. Firsov, Science 306, 666 (2004).
A.H.C. Neto, F. Guinea, N.M.R. Peres, K.S. Novoselov, and A.K. Geim, Rev. Mod. Phys. 81, 109 (2009).
L. Dössel, L. Gherghel, X. Feng, and K. Müllen, Angew. Chem. Int. Ed. 50, 2540 (2011).
P. Jangid, D. Pathan, and A. Kottantharayil, Carbon 132, 65 (2018).
Y.W. Son, M.L. Cohen, and S.G. Louie, Phys. Rev. Lett. 97, 216803 (2006).
N.L. Teradal, A.K. Satpati, and J. Seetharamappa, J. Electroanal. Chem. 797, 89 (2017).
T.C. Lin, Y.S. Li, W.H. Chiang, and Z. Pei, Biosens. Bioelectron. 89, 511 (2017).
P. Tseng, C.H. Chen, S.A. Hsu, and W.J. Hsueh, Phys. Lett. A 382, 1427 (2018).
J. Kusuma, R.G. Balakrishna, S. Patil, M.S. Jyothi, H.R. Chandan, and R. Shwetharani, Sol. Energy Mater. Sol. Cells 183, 211 (2018).
Y. Zhu, X. Li, Q. Cai, Z. Sun, G. Casillas, M. Jose-Yacaman, R. Verduzco, and J.M. Tour, J. Am. Chem. Soc. 134, 11774 (2012).
A. Ramasubramaniam, Phys. Rev. B 81, 245413 (2010).
J. Smotlacha and R. Pincak, Phys. Lett. A 382, 846 (2018).
A. Mohammadi and S. Haji-Nasiri, Phys. Lett. A 382, 1040 (2018).
O. Leenaert, B. Partoens, and F.M. Peeters, Phys. Rev. B 77, 125416 (2008).
W. Wang and G. Zhao, Solid State Commun. 166, 6 (2013).
P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G.L. Chiarotti, M. Cococcioni, I. Dabo, A.D. Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A.P. Seitsonen, A. Smogunov, P. Umari, and R.M. Wentzcovitch, J. Phys. Condens. Mater. 21, 395502 (2009).
S.S. Yu, W.T. Zheng, Q.B. Wen, and Q. Jiang, Carbon 46, 537 (2008).
J.Y. Dai and J.M. Yuan, J. Phys. Condens. Mat. 22, 225501 (2010).
K. Nakada and M. Fujita, Phys. Rev. B 54, 17954 (1996).
Acknowledgements
This work is supported by the Natural Science Foundation of Inner Mongolia (No. 2016BS0107), the National Natural Science Foundation of China (no. 11464034) and a scientific research project of Inner Mongolia University for Nationalities (No. NMDGP1718).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Zhao, C. & Li, P. Tuning the Electronic Properties of Graphene Oxide Nanoribbons Through Different Oxygen Doping Configurations. J. Electron. Mater. 47, 7093–7098 (2018). https://doi.org/10.1007/s11664-018-6638-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6638-2