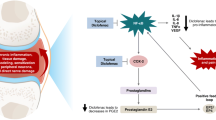
Abstract
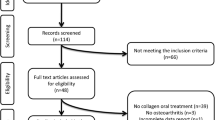
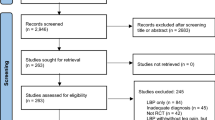
A systematic review of clinical applications of MSCs in the treatment of knee osteoarthritis (OA). Twenty-three papers were included in the final analysis (10 on BMAC and 13 on SVF). Of these, only 4 were randomised controlled trials (RCTs). The Coleman Score, used for bias risk evaluation, revealed an overall poor quality of the studies. In terms of clinical application, despite the apparent safety and the short-term positive clinical outcomes, clinicians reported different preparation and administration methods. The available literature is not sufficient to make any recommendation on the use of either product in clinical practice, even though they have both shown to be safe and have some short-term beneficial effects.
Similar content being viewed by others
Bibliografia
Poole AR (2003) What type of cartilage repair are we attempting to attain? J Bone Jt Surg, Am 85-A(Suppl):40–44
Richards MM, Maxwell JS, Weng L et al. (2016) Intra-articular treatment of knee osteoarthritis: from anti-inflammatories to products of regenerative medicine. Phys Sportsmed 44(2):101–108
Heijink A, Gomoll AH, Madry H et al. (2012) Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20(3):423–435
Vos T, Flaxman AD, Naghavi M et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196
Yoshimura K, Shigeura T, Matsumoto D et al. (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208(1):64–76
Verbus EA, Kenyon JD, Sergeeva O et al. (2017) Expression of miR-145-5p during chondrogenesis of mesenchymal stem cells. J Stem Cell Res (Overl Park) 1(3):1–10
DiMarino AM, Caplan AI, Bonfield TL (2013) Mesenchymal stem cells in tissue repair. Front Immunol 4:201
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213(2):341–347
Wu L, Cai X, Zhang S et al. (2013) Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J Cell Physiol 228(5):938–944
Filardo G, Madry H, Jelic M et al. (2013) Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 21(8):1717–1729
US Food and Drug Administration (2018) Regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use. Guidance for industry and Food and Drug Administration Staff www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM585403.pdf. Accessed February 2018
de Girolamo L, Kon E, Filardo G et al. (2016) Regenerative approaches for the treatment of early OA. Knee Surg Sports Traumatol Arthrosc 24(6):1826–1835
Pak J, Lee J, Pak N et al. (2018) Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status. Int J Mol Sci 19(7):2146
Moher D, Liberati A, Tetzlaff J, Altman DG (the PRISMA Group) (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Kon E, Verdonk P, Condello V (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee systematic clinical data review. Am J Sports Med 2009:156–166
Themistocleous GS, Chloros GD, Kyrantzoulis IM et al.(2018) Effectiveness of a single intra-articular bone marrow aspirate concentrate (BMAC) injection in patients with grade 3 and 4 knee osteoarthritis. Heliyon 4(10):e00871
Shaw B, Darrow M, Derian A (2018) Short-term outcomes in treatment of knee osteoarthritis with 4 bone marrow concentrate injections. Clin Med Insights Arthritis Musculoskelet Disord. https://doi.org/10.1177/1179544118781080
Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, Pascual-Garrido C (2018) Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: a cohort study. Phys Med Rehabil J 10(12):1353–1359
Shapiro SA, Arthurs JR, Heckman MG et al. (2019) Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 10(4):432–443
Sampson S, Vincent H, Aufiero D (2016) Intra-articular bone marrow concentrate injection protocol: short-term efficacy in osteoarthritis. Regen Med 11:511–520
Centeno CJ, Al-Sayegh H, Bashir J et al. (2015) A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord 16:258
Centeno C, Pitts J, Al-Sayegh H, Freeman M (2014) Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. BioMed Res Int 2014:370621
Kim J-D, Lee GW, Jung GH et al. (2014) Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 24:1505–1511
Vad V, Barve R, Linnell E, Harrison J (2016) Knee osteoarthritis treated with percutaneous chondral-bone interface optimization: a pilot trial. Surg Sci 7:1–12
Hernigou P, Auregan JC, Dubory A et al. (2018) Subchondral stem cell therapy versus controlateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int Orthop 42:2563–2571
Hudetz D, Bori I, Rod E et al. (2017) The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes (Basel) 8(10):E270
Yokota N, Yamakawa M, Shirata T, Kimura T (2017) Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther 6:108–112
Jones IA, Wilson M, Togashi R et al. (2018) A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord 19(1):383
Roato I, Belisario DC, Compagno M et al. (2018) Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. Int Orthop 43(1):15–23
Bansal H, Comella K, Leon J et al. (2017) Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 15(1):141
Bui KH, Duong TD, Nguyen NT et al. (2014) Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 2014(1):02
Koh Y, Kwon O, Kim Y, Choi Y (2014) Comparative outcomes of open-wedge high tibial osteotomy mesenchymal stem cell treatment: a prospective study. Arthroscopy 30(11):1453–1460
Hong Z, Chen J, Zhang S et al. (2018) Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 43(5):1123–1134
Kim YS, Hospital YS, Suh DS et al. (2015) Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med 43(1):176–185
Koh YG, Choi YJ, Kwon OR (2014) Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 42(7):1628–1637
Kwon YK (2013) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23(5):1308–1316
Nguyen PD, Tran TD-X, Nguyen HT-N et al. (2016) Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl Med 6:187–195
Koh Y-G, Jo S-B, Kwon O-R et al. (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29(4):748–755
Di Matteo B, Kon E (2019) Editorial commentary: biologic products for cartilage regeneration—time to redefine the rules of the game? Arthroscopy 35(1):260–261
Hadley CJ, Shi WJ, Murphy H et al. (2019) The clinical evidence behind biologic therapies promoted at annual orthopaedic meetings: a systematic review. Arthroscopy 35(1):251–259
Di Matteo B, Marcacci M, Kon E (2018) Letter to the editor concerning the article: “Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial” (Hong et al. International Orthopaedics doi:10.1007/s00264-018-4099-0). Int Orthop 43(3):751–752
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100(9):1249–1260
Zuk PA, Zhu M, Ashjian P et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Aust L, Devlin B, Foster SJ et al. (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6(1):7–14
Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M et al. (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8(2):166–177
Vezzani B, Shaw I, Lesme H et al. (2018) Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl Med 2018:876–886
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflitto di interesse
Gli autori F. Vandenbulcke, G. Beltrame, N.D. Vitale, B. Di Matteo e E. Kon dichiarano di non avere alcun conflitto di interesse.
Consenso informato e conformità agli standard etici
Tutte le procedure descritte nello studio e che hanno coinvolto esseri umani sono state attuate in conformità alle norme etiche stabilite dalla dichiarazione di Helsinki del 1975 e successive modifiche. Il consenso informato è stato ottenuto da tutti i pazienti inclusi nello studio.
Human and Animal Rights
L’articolo non contiene alcuno studio eseguito su esseri umani e su animali da parte degli autori.
Rights and permissions
About this article
Cite this article
Vandenbulcke, F., Beltrame, G., Vitale, N.D. et al. Le cellule staminali: impiego clinico. LO SCALPELLO 33, 237–242 (2019). https://doi.org/10.1007/s11639-019-00345-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11639-019-00345-9