Abstract
Background
Connected devices that allow people with diabetes to monitor their blood glucose levels remotely with data visualization have been shown to improve self-care behavior in diabetes management. However, their effectiveness and usability for a low-middle-income, racially diverse population are unknown.
Objective
This study aims to evaluate the effects of remote telemonitoring with team-based management on people with uncontrolled type 2 diabetes.
Design
This was a pragmatic 52-week cluster-randomized controlled study among 11 primary care government practices in Malaysia.
Participants
People with type 2 diabetes aged 18 and above, who had hemoglobin A1c ≥ 7.5% but less than 11.0% within the past 3 months and resided in the state of Selangor.
Intervention
The intervention group received home gluco-telemonitors and transmitted glucose data to a care team who could adjust therapy accordingly. The team also facilitated self-management by supporting participants to improve medication adherence, and encourage healthier lifestyle and use of resources to reduce risk factors. Usual care group received routine healthcare service.
Main Measure
The primary outcome was the change in HbA1c at 24 weeks and 52 weeks. Secondary outcomes included change in fasting plasma glucose, blood pressure, lipid levels, health-related quality of life, and diabetes self-efficacy.
Results
A total of 240 participants were recruited in this study. The telemonitoring group reported larger improvements in glycemic control compared with control at the end of study (week 24, − 0.05%; 95% CI − 0.10 to 0.00%) and at follow-up (week 52, − 0.03%; − 0.07 to 0.02%, p = 0.226). Similarly, no differences in other secondary outcomes were observed, including the number of adverse events and health-related quality of life.
Conclusion
This study indicates that there is limited benefit of replacing telemedicine with the current practice of self-monitoring of blood glucose. Further innovative methods to improve patient engagement in diabetes care are needed.
Trial Registration
ClinicalTrials.gov identifier: NCT02466880
Similar content being viewed by others
INTRODUCTION
Diabetes is a major public health concern and one of the four priority non-communicable diseases identified for action by the World Health Organization (WHO). In 2014, an estimated 422 million people worldwide had diabetes, and this figure is expected to increase to 552 million people by 2030.1 This increase is mainly driven by a rise in the number of people diagnosed with diabetes especially in low- and middle-income countries.2 Diabetes-related deaths have been increasing steadily, with an estimated 1.5 million deaths in 2012.3 Diabetes and its complications bring about detrimental effects as well as substantial economic burden on individuals, households, and governments.4 As such, innovative solutions are recommended to supplement conventional diabetes management strategies.
Decades of research have shown that interventions such as pharmacological therapies, physical activities, and diet control, as well as control of blood pressure and lipid can improve clinical outcomes of people with diabetes. A recent systematic review concluded that diabetes management can be further strengthened through improvements in chronic disease management alongside patient- and provider-mediated quality improvement strategies such as clinicians’ education and audit or patients’ education and reminders.5
Self-monitoring of blood glucose is a useful adjunct to team-based care for many people with diabetes. Telemonitoring, or the use of electronic technology to reduce geographical barriers while enabling access to tailored treatment, can be useful as actionable information can be provided to patients with poor management of blood glucose levels. Several studies have shown the potential of telemedicine in chronic disease management such as asthma and diabetes in developed countries.6,7,8,9,10 However, limited evidence was found on the potential use of telemedicine in the healthcare system of a developing country.11 The aim of this study was to evaluate the effects of telemedicine on people with type 2 diabetes whose glycemic levels are uncontrolled.
RESEARCH DESIGN AND METHODS
Study Design, Setting, and Participants
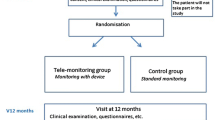
A 52-week cluster-randomized controlled trial was conducted in eleven primary healthcare centers in the Klang Valley, in Malaysia, a middle-income country. These clinics are part of the Ministry of Health’s health clinic network and serve the districts of Klang and Petaling with a total population of 2.56 million. Eligible clinics were randomized using a computer-generated randomization treatment code by the first author and allocated to either telemonitoring (TG) or usual care (UC). Clinicians and participants had no access to the list but could not be blinded to group allocation after randomization. The study’s protocol, design, and rationale have been published previously.12 Ethics approval was obtained from the Medical Research and Ethics Committee, Malaysia (NMRR-14-1368-22943), and Monash University Research Ethics Committee (CF15/1073-2015000502).
Registers of patients in participating primary healthcare clinics were screened to identify eligible participants. Selection criteria included the following: people with type 2 diabetes at least 6 months prior to study enrolment, aged between 18 and 75 years old, currently residing in the state of Selangor without plans to leave for the next 12 months, and HbA1c levels of ≥ 7.5% but less than 11.0% within the past 3 months. All participants provided written informed consent at the beginning of clinic visit. Recruitment occurred between April 2015 and June 2016, and the study was completed in August 2017.
Participants attended clinics for baseline screening and enrollment, and at weeks 4, 12, 24, and 52 for follow-up. Research assistants who were blinded to the allocation of participants, collected baseline information including blood glucose levels (HbA1c and fasting plasma glucose), lipid levels, blood pressure, height and weight, education level, and marital status. The number and types of medications were also obtained.
Intervention and Usual Care
During the 52-week study period, all participants were eligible to receive any services offered by the local healthcare providers, which could include group-based diabetes education, dietary counselling, and medication reviews by the pharmacists. UC participants continued to receive care from their doctors as they had in the past and monitor their blood glucose as required using a glucometer (OneTouch Select, Johnson & Johnson).
TG participants received a gluco-telemeter (MyGlucoHealth, Entra Health System) that automatically uploaded any blood glucose readings to an online portal. Participants were instructed to transmit up to 6 glucose readings weekly (3 pre-prandial and 3 post-prandial) to a central server. During the 6-month intervention, TG participants received automated feedback on their glycemic and metabolic results. If 3 consecutive readings of either hypoglycemia (70 mg/dL or below) or hyperglycemia (200 mg/dL and above) were detected, a message would be transmitted to inform the attending doctor or researcher, who would then recommend treatment changes based upon an algorithm. Briefly, no medication changes were suggested if 75% of glucose readings targets were within the 200 mg/dL target. If fewer than 75% of readings met the glucose targets, treatment intensification was recommended to the doctor. The decision to make regimen changes was at the sole discretion of the doctor. Drug dosage was adjusted by the clinician if a participant experienced adverse effects.
Participants in the TG arm also received monthly communications from the research team on self-management skills, blood glucose control, and the importance of medication adherence aimed at educating and motivating patients. Participants also attended a clinic visit at weeks 4, 12, and 24, where additional diabetes self-management education was given. During the months 7 through 12, all participants attended their usual clinic visits for follow-up (usually schedule every 3 months or more frequent as required by the patient) as part of routine care but no longer received any support from the study team.
Outcomes
The primary outcome was the change in HbA1c levels at weeks 24 and 52 from baseline visit, measured by HbA1c from a single laboratory. Other outcomes included change in fasting plasma glucose, blood pressure, and lipid levels at each time point. We also assessed health-related quality of life using the EuroQol-5D questionnaire13 while self-efficacy was assessed using the Michigan Diabetes Knowledge questionnaire.14 Emotional distress and functioning in diabetes were measured using the Problem Area in Diabetes (PAID) questionnaire.15
Direct Program Cost Estimate
Three broad categories of costs were included: intervention implementation costs, patients’ incurred costs, and clinic incurred costs. These costs were captured from a societal perspective and analyzed over a 1-year period in accordance with the study which delivered an intervention over a period of up to 1 year. All participants provided self-reported information regarding their healthcare expenditure including cost of clinic visits (physiotherapist, dietician, surgeon, or stoma nurse), and medications. Intervention implementation costs included telemonitoring devices and consumables as well as laboratory tests. Cost category included infrastructure, equipment operation and maintenance, laboratory, pharmacy, staff salary, and administration costs.16 All costs were expressed in USD according to the average exchange rate of USD1 = MYR4.301 while cost from previous years was inflated to the base year values. No discounting was done as the study did not exceed 1 year.
Statistical Analysis
Sample size was based on the primary outcome and was calculated based upon an 80% power to detect a 0.5% difference in the primary outcome at 0.05 significance level, after adjusting for 20% drop-outs. For all analyses, all participants were analyzed according to their group (intention-to-treat, ITT). Baseline characteristics were compared using t test for continuous variable or chi-square test for categorical variable. To account for missing data at each time point, imputation was performed assuming no treatment response. For all comparison, we used a multivariate general linear model, which controlled for age, gender, education level, body weight, marital status, and diabetes duration. The model predicted from the treatment group by time interaction and included a random intercept for clinics, using all available data from baseline and follow-up time points. Secondary outcomes were analyzed in a similar manner as primary outcome. The adjusted intervention effects that were estimated with these models were reported together with their standard deviation and p values. Two sensitivity analyses were also performed for the primary outcome, whereby we repeated the analysis using data without imputation (per-protocol) as well as without any adjustment. The number of glucose test patterns was summarized from data gathered from the remote uploads in the TG group as well as participants’ diary. All analyses were conducted using IBM SPSS version 20 (Armonk, NY).
RESULTS
Of the 1388 potentially eligible participants, 508 (36.6%) participants expressed interest and were screened (Fig. 1). Reasons for non-participation include the following: unable to commit (due to the long duration of intervention and transportation issues), did not have any mobile data plans or Internet access, and owning an incompatible smartphone. In total, 240 participants were enrolled, with 120 assigned to the TG group and 120 assigned to the UC group. At baseline, participants had a mean age of 56 years (TG 56.3 ± 8.6, UC 56.1 ± 9.2), 90% were married, and 55% were women. No significant differences were observed in the demographic characteristics of participants in the TG and UC groups (Table 1). In total, 208 (86.7%) participants provided follow-up data. Participant relocation, difficulty in attending follow-ups, family issues, and lack of willingness to complete the study were among the reasons given for participants’ attrition. No difference in baseline measures was observed between those who completed the study and those who discontinued (Appendix Table 1).
Primary Outcome
At week 24, both groups showed significant reduction in the HbA1c levels from baseline (Fig. 2a). There was a marginal difference from baseline to 24 weeks between both groups (− 0.05%; 95% CI − 0.10 to 0.00%), but this did not reach statistical significance. There was no evidence that telemedicine improved glycemic control at the end of the study (− 0.03%; − 0.07 to 0.02%; Table 2). This conclusion was unchanged in our sensitivity analyses with the per-protocol analyses or without imputation (Appendix Table 2). No significant differences were observed in the number of participants who achieved HbA1c target levels of < 7%. At the end of the study at week 52, a total of 23 (19.2%) participants in the TG group achieved HbA1c levels of < 7% compared with 21 (17.5%) participants in the UC group.
a Model estimated changes in mean hemoglobin A1cover time. The intervals represent pointwise 95% confidence interval for each group. b Weekly number of tests that were performed and reported by participants. Lines represent the locally weighted smoothing using a moving average across the observed test reported.
Secondary Outcomes
Evidence was lacking for any between-group differences in other outcomes measured at different time points, including patient-reported outcomes such as PAID, EQ-5D, Diabetes Knowledge Test, and cholesterol levels (Table 2). Medication adjustments were made to five participants throughout the study (three in UC and two in TG). Average number of self-blood glucose testing dropped consistently in both groups throughout the study, with the largest decrease after the study ended (Fig. 2b and Appendix Table 3).
Safety and Adverse Events
No adverse events or serious adverse events were reported during the intervention which were adjudicated to be study related.
Cost Analysis
Cost analysis conducted in this study was calculated as per group for the duration of the study. Analysis showed that telemonitoring for diabetes management costs a total of USD19,443.2 compared with conventional diabetes management which costs USD15,450.51 (Appendix Table 3). The increase in cost was due to the follow-up cost by the researcher and higher cost of purchasing a web-based glucometer and its strips. However, the use of a telemonitoring strategy reduced the cost of ward admissions by USD744.66 at the end of the study compared with usual care. Similarly, cost of consultant visit was USD44.91 lower compared with the usual care group.
Interpretation
In this study, we did not identify any clinical or statistically significant difference in glycemic control between participants who received remote telemonitoring with team-based management compared with usual care. Results of this study corresponded s with those of other studies which showed negligible effects of telemedicine.17, 18 It is possible that more intense intervention during follow-up and additional appointments could have strengthened the self-management and behavior change, further improving glycemic control in the intervention group. However, the present study was not sufficiently powered to determine the effectiveness in these clinical situations, such as weekly versus daily testing or impact of medication changes.
The combination of home glucose telemonitoring with coaching for people with diabetes has been examined by several high-quality studies. The Mobile Diabetes Intervention Study which examined the use of mobile application coaching with decision support found that such intervention could reduce HbA1c by 1.2% compared with usual care, with no differences observed in other patient-reported diabetes outcomes.19 Similarly, Nicolucci and colleagues found that home telemonitoring reduced HbA1c by 0.33% during the 6-month study period.20 Our initial pilot study on patients fasting during Ramadan also noted that telemonitoring with coaching was effective in reducing HbA1c and hypoglycemia.21 However, our findings are contrary to those presented above. This could possibly be that the intervention was insufficient to engage patients effectively as noted by the number of medication changes and SMBG uploads made throughout the study. This was similarly revealed in the focus group discussion (data presented separately) whereby study nurses lamented the lack of cooperation from participants especially when they felt that their blood glucose was under control. Studies have suggested that for telemonitoring to be effective, both patients and clinicians need to be actively engaged in performing, interpreting, and acting on the results.11, 22, 23
Another possible reason for the lack of changes observed in this study could be the low engagement among participants in this study. While we had requested that participants perform up to 6 test per week, the number of uploads were far below our expectations, averaging between 0.75 and 0.86 uploads/patient at week 4 and tapering to 0.70 to 0.79 uploads at the end of the trial at week 24 (Appendix Table 3). This was despite the constant reminders and messages which were sent to all participants in the TG group. While various reviews and meta-analyses have suggested that telemedicine can potentially improve glycemic control, this could be due to the ideal research setting compared with the pragmatic design in this study. In addition, most studies are often conducted in high-income countries, where health and technology literacy among individuals are higher compared with the general population in Malaysia where there was very low health and technology literacy (data presented separately). As such, future diabetes program should focus on the appropriate development, adaptation, and implementation of program that are context-specific and tailored to the cultural, religious, and socioeconomic needs of the target communities examined, to ensure the sustainability of targeted intervention.
Other barriers to implementation include problems with end-user acceptance of technology, such as the lack of familiarity with the technology as well as necessary infrastructure such as internet access and data plans to support the implementation. Cost was another major barrier and key impediment to technology implementation. In our cost analysis, we found that the setup and implementation cost contributed to 77% of our total direct cost, mainly driven by equipment cost as well as personnel cost for telemedicine implementation. This concurs with other similar studies conducted in many other low-middle-income countries,23,24,25 where cost of medical services is minimal compared with many high-income countries. Nevertheless, we believe that this situation will change in the foreseeable future as technology becomes cheaper which will make telemedicine a cost-effective alternative irrespective of setting.
As the first large pragmatic trial in a middle-income country conducted in South East Asian region, results of this study provide healthcare professionals and policymakers with evidence on the use of telemedicine in diabetes. Results of the current study noted that telemonitoring was more expensive, and contributed to the need to procure telemonitoring devices and consumables, which was not imposed on the control group. This point was similarly noted during our focus group discussion with healthcare providers, who had doubts on the cost-effectiveness of the intervention. It may be possible to reduce the total program cost through better targeting of patients, individual tailoring of intervention, and further negotiation with volume discounts. In addition, this cost did not take into account the long-term cost saving from averting any diabetes-related complications, such as microvascular as well as macrovascular complications, which could be substantial.
Strengths and Limitation of this Study
Results of this study should be interpreted in view of the strengths and limitation. Unique features and strengths of our study were the use of telemonitoring performed in a community setting, using a cluster design with an intervention of 6 months and extended follow-up at 12 months. Our study also further expands the knowledge in the literature as only few studies had explored the use of telemedicine from middle-income countries11 where there is a rising prevalence of diabetes. This study was conducted in a government healthcare setting, where the majority of diabetes care in the nation was provided.
The present study has several limitations. There was a large proportion of unemployed participants in this study and many were of the older age group; hence, participants required additional coaching sessions which increased the cost of intervention. As this study was conducted in the suburban and rural areas, limited internet connectivity as well as use of older smartphones (due to older firmware) led to a high rejection rate. As with any complex intervention, we could not separate how much of the intervention effect was attributable to telemonitoring or health coaching. However, as all people with diabetes will now receive health coaching and education as part of standard of care, it is likely that the impact on glycated hemoglobin can be attributable largely to the effects of telemontirong intervention. Another limitation was the lack of information on medication titration and escalation by the clinicians during the study. Data for cost analysis was obtained either from self-reporting by participants or from published literature, which may have led to an over/under-estimation of the study cost. Lastly, there was a lack of information on the long-term effects after 12 months, which could be addressed with a planned long-term follow-up in the future.
In summary, the inclusion of technology has been touted as the game changer for patients and management of chronic disease management. Results of this study showed that the addition of telemedicine in replacement of self-monitoring in diabetes care had limited clinical benefits in improving glycemic control. These findings suggest that further research on means to improve patient engagement is needed to optimize healthcare services while maintaining high-quality standards.
References
Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530.
Organization WH. Global Report on Diabetes. In: Organization WH, ed. Geneva: World Health Organization; 2016.
Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015;33(8):811–831.
Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–2261.
Lee JY, Lee SW, Nasir NH, How S, Tan CS, Wong CP. Diabetes telemonitoring reduces the risk of hypoglycaemia during Ramadan: a pilot randomized controlled study. Diabet Med. 2015;32(12):1658–1661.
Wild SH, Hanley J, Lewis SC, et al. Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med. 2016;13(7):e1002098.
Sood A, Watts SA, Johnson JK, Hirth S, Aron DC. Telemedicine consultation for patients with diabetes mellitus: a cluster randomised controlled trial. J Telemed Telecare. 2017:1357633X17704346.
Paré G, Jaana M, Sicotte C. Systematic Review of Home Telemonitoring for Chronic Diseases: The Evidence Base. J Am Med Inform Assoc. 2007;14(3):269–277.
Pare G, Moqadem K, Pineau G, St-Hilaire C. Clinical effects of home telemonitoring in the context of diabetes, asthma, heart failure and hypertension: a systematic review. J Med Internet Res. 2010;12(2):e21.
Lee SWH, Chan CKY, Chua SS, Chaiyakunapruk N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: A systematic review and network meta-analysis. Sci Rep. 2017;7(1):12680.
Lee JY, Chan CKY, Chua SS, et al. Intervention for Diabetes with Education, Advancement and Support (IDEAS) study: protocol for a cluster randomised controlled trial. BMC Health Serv Res. 2016;16:524.
Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13(9):873–884.
Al-Qazaz HK, Hassali MA, Shafie AA, Sulaiman SAS, Sundram S. The 14-item Michigan Diabetes Knowledge Test: translation and validation study of the Malaysian version. Pract Diabetes Int. 2010;27(6):238-241a.
Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale: An evaluation of its clinical utility. Diabetes Care. 1997;20(5):760–766.
Ezat SE, Na A, Mn A, Bs S. Economic burden of diabetic care in government Health Facilities in Selangor. Vol 152009.
Young LA, Buse JB, Weaver MA, et al. Glucose Self-monitoring in Non-Insulin-Treated Patients With Type 2 Diabetes in Primary Care Settings: A Randomized Trial. JAMA Intern Med. 2017;177(7):920–929.
Katalenich B, Shi L, Liu S, et al. Evaluation of a Remote Monitoring System for Diabetes Control. Clin Ther. 2015;37(6):1216–1225.
Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934–1942.
Nicolucci A, Cercone S, Chiriatti A, Muscas F, Gensini G. A Randomized Trial on Home Telemonitoring for the Management of Metabolic and Cardiovascular Risk in Patients with Type 2 Diabetes. Diabetes Technol Ther 2015;17(8):563–570.
Lee JY, Wong CP, Tan CSS, Nasir NH, Lee SWH. Telemonitoring in fasting individuals with Type 2 Diabetes Mellitus during Ramadan: A prospective, randomised controlled study. Sci Rep. 2017;7(1):10119.
Lee SW, Ooi L, Lai YK. Telemedicine for the Management of Glycemic Control and Clinical Outcomes of type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled studies. Front Pharmacol. 2017;8:330.
Lee JY, Lee SWH. Is telemedicine cost effectiveness for diabetes management: A systematic review. Diabetes Technol Ther. 2018;20(7):492–500.
Bagayoko CO, Traoré D, Thevoz L, et al. Medical and economic benefits of telehealth in low- and middle-income countries: results of a study in four district hospitals in Mali. BMC Health Serv Res. 2014;14(Suppl 1):S9-S9.
McConnell KA, Krisher LK, Lenssen M, Bunik M, Bunge Montes S, Domek GJ. Telehealth to Expand Community Health Nurse Education in Rural Guatemala: A Pilot Feasibility and Acceptability Evaluation. Front Public Health. 2017;5(60).
Acknowledgments
The authors would like to thank health clinics and patients who participated in this study.
Funding
Funding of this study was through the e-Science fund from the Ministry of Science, Technology and Innovation, Malaysia (03-02-10-SF0238 (MOSTI)).
Author information
Authors and Affiliations
Contributions
All authors named contributed substantially to the document. JYL collected data, interpreted the results, and wrote the draft manuscript. SWHL obtained the funding, designed the study, and provided support in editing the manuscript. CKYC, SSC, CJN, TP, and KKCL contributed to the study design and reviewed the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval was obtained from the Medical Research and Ethics Committee, Malaysia (NMRR-14-1368-22943), and Monash University Research Ethics Committee (CF15/1073-2015000502). All participants provided written informed consent at the beginning of clinic visit.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Lee, J.Y., Chan, C.K.Y., Chua, S.S. et al. Telemonitoring and Team-Based Management of Glycemic Control on People with Type 2 Diabetes: a Cluster-Randomized Controlled Trial. J GEN INTERN MED 35, 87–94 (2020). https://doi.org/10.1007/s11606-019-05316-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-019-05316-9