Abstract
Introduction
Oxaliplatin is part of pancreatic cancer therapy in the FOLFIRINOX or GEMOX/XELOX regimen. DNA damage repair is one of the factors responsible for oxaliplatin resistance that eventually develops in this cancer. Triptolide/Minnelide has been shown to be effective against pancreatic cancer in preclinical trials. In this study, we evaluated the efficacy of combination of triptolide and oxaliplatin against pancreatic cancer.
Methods
Highly aggressive pancreatic cancer cells (MIA PaCa-2 and PANC-1) were treated with oxaliplatin (0–10 μM), low-dose triptolide (50 nM), or a combination of both for 24–48 h. Cell viability, apoptosis, and DNA damage were evaluated by appropriate methods. Nucleotide excision repair pathway components were quantitated using qPCR and Western blot. Combination of low doses of Minnelide and oxaliplatin was tested in an orthotopic murine model of pancreatic cancer.
Results
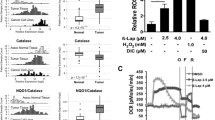
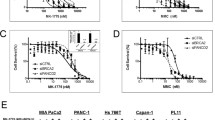
Proliferation of pancreatic cancer cells was markedly inhibited by combination treatment. Triptolide potentiated apoptotic cell death induced by oxaliplatin and sensitized cancer cells towards oxaliplatin-induced DNA damage by suppressing the oxaliplatin-induced DNA damage repair pathway. Combination of low doses of Minnelide and oxaliplatin inhibited tumor progression by inducing significant apoptotic cell death in these tumors.
Conclusions
Combination of low doses of Minnelide and oxaliplatin has immense potential to emerge as a novel therapeutic strategy against pancreatic cancer.








Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29.
Wolfgang CL, Herman JM, Laheru DA et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013; 63: 318–348.
Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703.
Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825.
Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22: 7265–7279.
Reed E. ERCC1 and clinical resistance to platinum-based therapy. Clin Cancer Res 2005; 11: 6100–6102.
Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol 2011; 18: 18–25.
Chaney SG, Vaisman A. Specificity of platinum-DNA adduct repair. J Inorg Biochem 1999; 77: 71–81.
McNeil EM, Melton DW. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res 2012; 40: 9990–10004.
Li Z, Qing Y, Guan W et al. Predictive value of APE1, BRCA1, ERCC1 and TUBB3 expression in patients with advanced non-small cell lung cancer (NSCLC) receiving first-line platinum-paclitaxel chemotherapy. Cancer Chemother Pharmacol 2014; 74: 777–786.
Kuhlmann JD, Wimberger P, Bankfalvi A et al. ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin Chem 2014; 60: 1282–1289.
Mancuso A, Sacchetta S, Saletti PC et al. Clinical and molecular determinants of survival in pancreatic cancer patients treated with second-line chemotherapy: results of an Italian/Swiss multicenter survey. Anticancer Res 2010; 30: 4289–4295.
Phillips PA, Dudeja V, McCarroll JA et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res 2007; 67: 9407–9416.
Chugh R, Sangwan V, Patil SP et al. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 2012; 4: 156ra139.
Zhong YY, Chen HP, Tan BZ et al. Triptolide avoids cisplatin resistance and induces apoptosis via the reactive oxygen species/nuclear factor-kappaB pathway in SKOV3 platinum-resistant human ovarian cancer cells. Oncol Lett 2013; 6: 1084–1092.
Ho JN, Byun SS, Lee S et al. Synergistic antitumor effect of triptolide and cisplatin in cisplatin resistant human bladder cancer cells. J Urol 2015; 193: 1016–1022.
Liu Y, Xiao E, Yuan L, Li G. Triptolide synergistically enhances antitumor activity of oxaliplatin in colon carcinoma in vitro and in vivo. DNA Cell Biol 2014; 33: 418–425.
Zhu W, Li J, Wu S et al. Triptolide cooperates with cisplatin to induce apoptosis in gemcitabine-resistant pancreatic cancer. Pancreas 2012; 41: 1029–1038.
Li CJ, Chu CY, Huang LH et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett 2012; 319: 203–213.
Chen Z, Sangwan V, Banerjee S et al. Triptolide sensitizes pancreatic cancer cells to TRAIL-induced activation of the death receptor pathway. Cancer Lett 2014; 348: 156–166.
Borja-Cacho D, Yokoyama Y, Chugh RK et al. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. J Gastrointest Surg 2010; 14: 252–260.
Banerjee S, Kong D, Azmi AS et al. Restoring sensitivity to oxaliplatin by a novel approach in gemcitabine-resistant pancreatic cancer cells in vitro and in vivo. Int J Cancer 2011; 128: 1240–1250.
Mujumdar N, Mackenzie TN, Dudeja V et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology 2010; 139: 598–608.
Seetharam RN, Sood A, Basu-Mallick A et al. Oxaliplatin resistance induced by ERCC1 up-regulation is abrogated by siRNA-mediated gene silencing in human colorectal cancer cells. Anticancer Res 2010; 30: 2531–2538.
Kuo LJ, Yang LX. Gamma-H2AX—a novel biomarker for DNA double-strand breaks. In Vivo 2008; 22: 305–309.
Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 2010; 24: 679–686.
Ivashkevich A, Redon CE, Nakamura AJ et al. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 2012; 327: 123–133.
Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol 2012; 920: 613–626.
Philip PA. Gemcitabine and platinum combinations in pancreatic cancer. Cancer 2002; 95: 908–911.
Isayama H, Nakai Y, Yamamoto K et al. Gemcitabine and oxaliplatin combination chemotherapy for patients with refractory pancreatic cancer. Oncology 2011; 80: 97–101.
Katopodis O, Souglakos J, Stathopoulos E et al. Frontline treatment with gemcitabine, oxaliplatin and erlotinib for the treatment of advanced or metastatic pancreatic cancer: a multicenter phase II study of the Hellenic Oncology Research Group (HORG). Cancer Chemother Pharmacol 2014; 74: 333–340.
Sancar A, Reardon JT. Nucleotide excision repair in E. coli and man. Adv Protein Chem 2004; 69: 43–71.
Aboussekhra A, Biggerstaff M, Shivji MK et al. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 1995; 80: 859–868.
Camenisch U, Dip R, Schumacher SB et al. Recognition of helical kinks by xeroderma pigmentosum group A protein triggers DNA excision repair. Nat Struct Mol Biol 2006; 13: 278–284.
Arnould S, Hennebelle I, Canal P et al. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer 2003; 39: 112–119.
Prewett M, Deevi DS, Bassi R et al. Tumors established with cell lines selected for oxaliplatin resistance respond to oxaliplatin if combined with cetuximab. Clin Cancer Res 2007; 13: 7432–7440.
Xia Y, Hu C, Zhang M et al. [Relationship between ERCC1 expression and cisplatin intervention in human lung adenocarcinoma cell lines]. Zhongguo Fei Ai Za Zhi 2007; 10: 362–365.
Li Q, Yu JJ, Mu C et al. Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res 2000; 20: 645–652.
Wei KK, Jiang L, Wei YY et al. The prognostic value of ERCC1 expression in gastric cancer patients treated with platinum-based chemotherapy: a meta-analysis. Tumour Biol 2014.
Li S, Wu J, Chen Y et al. ERCC1 expression levels predict the outcome of platinum-based chemotherapies in advanced bladder cancer: a meta-analysis. Anticancer Drugs 2014; 25: 106–114.
Milovic-Kovacevic M, Srdic-Rajic T, Radulovic S et al. Expression of ERCC1 protein in biopsy specimen predicts survival in advanced ovarian cancer patients treated with platinum-based chemotherapy. J BUON 2011; 16: 708–714.
Soo RA, Yong WP, Innocenti F. Systemic therapies for pancreatic cancer—the role of pharmacogenetics. Curr Drug Targets 2012; 13: 811–828.
Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007; 33: 9–23.
Li Q, Gardner K, Zhang L et al. Cisplatin induction of ERCC-1 mRNA expression in A2780/CP70 human ovarian cancer cells. J Biol Chem 1998; 273: 23419–23425.
Bonovich M, Olive M, Reed E et al. Adenoviral delivery of A-FOS, an AP-1 dominant negative, selectively inhibits drug resistance in two human cancer cell lines. Cancer Gene Ther 2002; 9: 62–70.
Kudo K, Gavin E, Das S et al. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene 2012; 31: 4718–4724.
Zhong X, Thornton K, Reed E. Computer based analyses of the 5′-flanking regions of selected genes involved in the nucleotide excision repair complex. Int J Oncol 2000; 17: 375–380.
Reed E, Dabholkar M, Thornton K et al. Evidence for in the appearance of mRNAs of nucleotide excision repair genes, in human ovarian cancer tissues. Oncol Rep 2000; 7: 1123–1128.
Alsaied OA, Sangwan V, Banerjee S et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery 2014; 156: 270–279.
Acknowledgments
This study was funded by NIH grants R01-CA170946 and CA124723 (to AKS); NIH grant R01-CA184274 (to SB); UAB/UMN SPORE in Pancreatic Cancer-P50 CA101955 (to SM); Katherine and Robert Goodale foundation support (to AKS); and Minneamrita Therapeutics LLC (to AKS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The University of Minnesota has a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS is the co-founder and the Chief Scientific Officer of this company. SB is a consultant with Minneamrita Therapeutics LLC. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
Additional information
Primary Discussant
Jeffrey B. Matthews, M.D. (Chicago, IL): Despite advances in surgical therapy for pancreatic cancer, we have made precious little impact on overall survival in this disease due in part to slow progress in identifying effective chemotherapeutic agents, or combinations of agents, against this difficult tumor. You have shown that Minnelide, a synthetic prodrug of triptolide, increased cell death and reduced drug resistance to oxaliplatin. These results are interesting and suggest a novel approach to combat resistance to platinum-based chemotherapy. Nevertheless, our enthusiasm is always tempered by past experience. The road to successful cancer drug development is unfortunately littered with compounds that were effective in cell lines and murine models only to fail in the human disease. We eagerly await the results of your Phase 1 clinical trial.
I have two questions for you. First, because triptolide is derived from a Chinese herb, I am wondering
1) Whether the concentrations used in your experiments and trials are similar to what would be achieved in its use as a traditional Chinese medicine? Are there any specific toxicities and side effects?
2) Regarding mechanism of action. In previous publications from your lab, you suggested that heat shock protein 70 was involved in the pro-apoptotic action of Minnelide, and other work suggests involvement of reactive oxidant species and NfKB signaling. In today’s work, you focus on the nucleotide excision repair pathway. Can you connect the dots for us? Is there a single molecular target for Minnelide that might explain both its own apoptotic action and its effect on platinum resistance?
Closing Discussant
Dr. Modi
1. The extracts of Tripterygium wilfordii hook F (TWHF) contain around 80 active components with triptolide being one of them. Therefore, the direct comparison between the concentrations of triptolide achieved by administration of various extracts of Chinese herbal medicine and Minnelide is not feasible.
In the Phase I clinical trial, 27 patients have been enrolled so far, with 24 evaluable for toxicity. The therapy has been generally well tolerated with the only common toxicity being hematologic, but one patient experienced reversible cerebellar dysfunction at the highest dose. Hematologic toxicity has been notable for rapid onset and rapid recovery from neutropenia—often within 2–3 days of dose interruption. We have used these reference doses obtained from the trial in our experiments.
2. The exact molecular target of triptolide in a cancer cell is still not completely known. The cellular pathways are an intricate network of interconnected signaling. We have previously shown that triptolide inhibits Sp1 activity leading to decreased NF-κB signaling and thereby decreased HSP 70 levels. AP1 has also been shown to regulate pro-survival pathways like NF-κB and vice versa. Promoter regions of AP1 are predicted to have Sp1 and NF-κB binding sites as well (www.genecards.org). So, it is possible that triptolide affects these upstream events that culminate into AP1 inhibition leading to triggering of apoptotic pathways and overcoming platinum resistance.
The proposed mechanism of action of how triptolide inhibits AP1.

Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
mRNA expression of genes not inhibited by TPL treatment. MIA PaCa-2 cells were treated with TPL 100 nM for 24 h and data is represented as fold change over untreated cells. (PDF 1671 kb)
Supplementary Figure 2
Equi-effective doses of paclitaxel (PXL) and rapamycin (Rapa) do not affect components of NER pathway demonstrating the pathway’s specificity as a resistance mechanism to DNA damaging agents like oxaliplatin (Ox). MIA PaCa-2 (a) and PANC-1 (b) cells were treated with ED25 doses of Ox (5 μM), PXL (9.1 nM) and Rapa (248.1 nM) for 24 h and mRNA levels of ERCC1, XPA, XPB, XPC, XPD, XPF and XPG were analyzed. Data is represented as fold change over untreated cells. (PDF 2932 kb)
Rights and permissions
About this article
Cite this article
Modi, S., Kir, D., Giri, B. et al. Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer. J Gastrointest Surg 20, 13–24 (2016). https://doi.org/10.1007/s11605-015-3000-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-3000-3