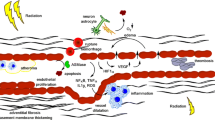
Abstract
Radiation therapy is a useful treatment for tumors and vascular malformations of the central nervous system. Radiation therapy is associated with complications, including leukoencephalopathy, radiation necrosis, vasculopathy, and optic neuropathy. Secondary tumors are also often seen long after radiation therapy. Secondary tumors are often benign tumors, such as hemangiomas and meningiomas, but sometimes malignant gliomas and soft tissue sarcomas emerge. We review the imaging findings of complications that may occur after brain radiation therapy.











Similar content being viewed by others
References
Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–70.
Rogers LR. Neurologic complications of radiation. Continuum (Minneap Minn). 2012;18:343–54.
Hoeffner EG. Central nervous system complications of oncologic therapy. Hematol Oncol Clin North Am. 2016;30:899–920.
Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639–51.
Ebi J, Sato H, Nakajima M, Shishido F, et al. Incidence of leukoencephalopathy after whole-brain radiation therapy for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:1212–7.
Zhong X, Huang B, Feng J, Yang W, Liu H. Delayed leukoencephalopathy of non-small cell lung cancer patients with brain metastases underwent whole brain radiation therapy. J Neurooncol. 2015;125:177–81.
Common Terminology Criteria for Adverse Events (CTCAE); National cancer institute Web site. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 24 June 2018.
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149–56.
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–84.
Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26:1967–72.
Rogers LR, Gutierrez J, Scarpace L, Schultz L, Ryu S, Lord B, Movsas B, et al. Morphologic magnetic resonance imaging features of therapy-induced cerebral necrosis. J Neurooncol. 2011;101:25–32.
Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005;26:1455–60.
Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25:201–9.
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30:552–8.
Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21:901–9.
Sundgren PC. MR spectroscopy in radiation injury. AJNR Am J Neuroradiol. 2009;30:1469–76.
Weybright P, Sundgren PC, Maly P, Hassan DG, Nan B, Rohrer S, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am J Roentgenol. 2005;185:1471–6.
Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35.
Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among 11C-Methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol. 2017;38:1520–7.
Kim YH, Oh SW, Lim YJ, Park CK, Lee SH, Kang KW, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112:758–65.
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010;66:486–91.
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61.
Young RJ, Gupta A, Shah AD, Graber JJ, Zhang Z, Shi W, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76:1918–24.
Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122:421–9.
Zhou L, Xing P, Zou L, Shen J, Tian Y, Lu X. Middle cerebral artery stenosis in patients with nasopharyngeal carcinoma after radiotherapy: the incidence of stenosis and the risk factors. Br J Radiol. 2016;89:20150815.
Kralik SF, Watson GA, Shih CS, Ho CY, Finke W, Buchsbaum J. Radiation-induced large vessel cerebral vasculopathy in pediatric patients with brain tumors treated with proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:817–24.
Gujral DM, Chahal N, Senior R, Harrington KJ, Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014;110:31–8.
Sattler MG, Vroomen PC, Sluiter WJ, Schers HJ, van den Berg G, Langendijk JA, et al. Incidence, causative mechanisms, and anatomic localization of stroke in pituitary adenoma patients treated with postoperative radiation therapy versus surgery alone. Int J Radiat Oncol Biol Phys. 2013;87:53–9.
Davis PC, Hoffman JC Jr, Pearl GS, Braun IF. CT evaluation of effects of cranial radiation therapy in children. AJR Am J Roentgenol. 1986;147:587–92.
Srinivasan KG, Ramprabananth S, Ushanandhini KP, Srividya S, Praveen Kumar M. Radiation-induced mineralizing microangiopathy in a case of recurrent craniopharyngioma. A case report. Neuroradiol J. 2010;23:412–5.
Kikuchi A, Maeda M, Hanada R, Okimoto Y, Ishimoto K, Kaneko T, et al. Moyamoya syndrome following childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:268–72.
Desai SS, Paulino AC, Mai WY, Teh BS. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys. 2006;65:1222–7.
Danesh-Meyer HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15:95–100.
Ferguson I, Huecker J, Huang J, McClelland C, Van Stavern G. Risk factors for radiation-induced optic neuropathy: a case-control study. Clin Exp Ophthalmol. 2017;45:592–7.
Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst. 2011;27:445–53.
Yamanaka R, Hayano A, Kanayama T. Radiation-induced meningiomas: an exhaustive review of the literature. World Neurosurg. 2017;97:635–44.
Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S. Radiation-induced meningioma. Neurosurg Focus. 2008;24:E7.
Di Giannatale A, Morana G, Rossi A, Cama A, Bertoluzzo L, Barra S. Natural history of cavernous malformations in children with brain tumors treated with radiotherapy and chemotherapy. J Neurooncol. 2014;117:311–20.
Strenger V, Sovinz P, Lackner H, Dornbusch HJ, Lingitz H, Eder HG, et al. Intracerebral cavernous hemangioma after cranial irradiation in childhood. Incidence and risk factors. Strahlenther Onkol. 2008;184:276–80.
Koike T, Yanagimachi N, Ishiguro H, Yabe H, Yabe M, Morimoto T, et al. High incidence of radiation-induced cavernous hemangioma in long-term survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant. 2012;18:1090–8.
Yamanaka R, Hayano A, Kanayama T. Radiation-induced gliomas: a comprehensive review and meta-analysis. Neurosurg Rev. 2016 Oct 5. [Epub ahead of print].
Elsamadicy AA, Babu R, Kirkpatrick JP, Adamson DC. Radiation-induced malignant gliomas: a current review. World Neurosurg. 2015;83:530–42.
Salvati M, Frati A, Russo N, Caroli E, Polli FM, Minniti G, et al. Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol. 2003;60:60–7.
Yamanaka R, Hayano A. Radiation-induced sarcomas of the central nervous system: a systematic review. World Neurosurg. 2017;98(818–828):e7.
Koshy M, Paulino AC, Mai WY, Teh BS. Radiation-induced osteosarcomas in the pediatric population. Int J Radiat Oncol Biol Phys. 2005;63:1169–74.
Debnam JM, Guha-Thakurta N, Mahfouz YM, Garden AS, Benjamin RS, Sturgis EM, et al. Radiation-associated head and neck sarcomas: spectrum of imaging findings. Oral Oncol. 2012;48:155–61.
Helms Clyde A, Tumors Malignant Bone, Helms Clyde A. Fundamentals of skeletal radiology. 3rd ed. London: Elsevier; 1994. p. 37–9.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical statement
This manuscript is a review article and does not contain any studies with human participants or animals performed by any of the authors.
About this article
Cite this article
Kanda, T., Wakabayashi, Y., Zeng, F. et al. Imaging findings in radiation therapy complications of the central nervous system. Jpn J Radiol 36, 519–527 (2018). https://doi.org/10.1007/s11604-018-0759-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0759-7